A new angle
Some of these cancers affect thousands of people. Others affect few. But, regardless of magnitude, the lessons we may learn may cure us all. — Brooke and Keith Desserich in “Notes Left Behind”
 Denise Downing with her daughter Caitlin.Image provided by Denise Downing
Denise Downing with her daughter Caitlin.Image provided by Denise Downing
Denise Downing remembers feeling incredulous and frightened as she sat with her husband, Jeff, in a doctor’s office on a January day in 2012. They just had heard a pediatric neuro-oncologist tell them that their daughter, 4-year-old Caitlin, had a rare brain tumor.
The doctor had diagnosed Caitlin with diffuse intrinsic pontine glioma, or DIPG. Denise Downing asked the specialist how many children had survived the disease and Caitlin’s chances of beating it.
The doctor said, “Zero.” Bewildered, Downing remembers asking, “Zero what?”
The doctor clarified: There were no survivors of DIPG.
Caitlin Downing died on November 11, 2012, nine months after her diagnosis. She was 5 years old.
Downloadable audio version
Each year in North America, DIPG kills between 250 and 300 children. Knowledge of the tumor stretches back a century, but a treatment, much less a cure, has been elusive. “There’s not a single approved drug,” says pediatric oncologist Oren Becher at Duke University.
Historically, many clinicians shied away from studying DIPG. “There was a huge disincentive for focusing on the disease. It’s rare. Funding was nonexistent. The outcome is dismal. Your chances of succeeding were horribly low,” says pediatric neurosurgeon Mark Souweidane of Weill Cornell Medical College.
That’s what the Downings discovered: Because DIPG is a rare disease, not much was known about it. Not much funding had gone toward studying it. There wasn’t much to do about it.
The pain and anger of having no recourse still ring in Downing’s voice. “We live in an age where we spend billions of dollars to send satellites into space, and we don’t even get upset when they get lost!” she says. “But 250 children die every year from a brain tumor, and we allow that to go on.”
But the tide is turning. Shortly after Caitlin was diagnosed, two scientific papers described the sequences of DNA from DIPG and other pediatric brain tumors. Researchers found mutations in the most unlikely location: histone genes.
Misregulation of proteins such as kinases and cell-cycle regulators is known to promote tumor growth. But not histones. “No one, absolutely no one, in their wildest dreams would have expected this,” says C. David Allis of The Rockefeller University, who studies these proteins around which a cell’s DNA winds like thread.
Since this unexpected finding, says Souweidane, the nearly nonexistent field of DIPG research has “exploded.” Molecular biologists like Allis suddenly are discovering that their curiosity-driven explorations of the genome’s packaging material have clinical implications.
We are going to help you enjoy your child’
The disease affects both girls and boys, usually between the ages of 4 and 11. Children with DIPG typically die within 12 months of their diagnosis.
The tumor sprouts out of a structure in the brainstem known as the pons. The pons is involved in eye movement, balance, hearing, facial expressions and other functions.
Caitlin’s first symptom was uncoordinated eye movement. It was the Sunday after Christmas 2011. The Downing family had gathered around the dining table. Denise Downing looked at her third child, whom she describes as a “girly-girl” who loved cheerleading, dancing, gymnastics and dolls all heavily doused in pink. The iris of the preschooler’s eye was drifting toward her nose.
Downing has relatives with lazy eye, so she offhandedly asked her husband, a family practice physician, if lazy eye is genetic. He said it was, and Downing thought no more of it. The next day, she noticed Caitlin’s eyes cross several times and that the child was holding her head oddly. Sure she was making something out of nothing, Downing carried on.
By Wednesday, it was clear to both Downing and Caitlin’s preschool teacher that the little girl was holding her head almost to the side. Still thinking lazy eye, Downing made an appointment with an ophthalmologist for the next day. The ophthalmologist assured Downing there was nothing wrong with Caitlin.
But something gnawed at Downing, and she decided to keep Caitlin with her that Thursday. Her daughter begged to go back to preschool, but Downing told her that she was going to have a fun day with Mommy with a trip to the mall and a McDonalds’ Happy Meal.
 Caitlin Downing died from DIPG in 2012.Image provided by Denise Downing.
Caitlin Downing died from DIPG in 2012.Image provided by Denise Downing.
I watched her,” says Downing. “Every hour, she seemed to be getting worse.”
Later in the day, Downing was home with her four children and talking to her oldest daughter, Courtney. Caitlin got up from in front of the television and tried to walk toward Downing. But she ran smack into Courtney and fell down. As she picked herself up, Caitlin, who Downing describes as sweet-tempered, yelled in anger at her sister for deliberately getting in her way. Both Courtney and Downing stared at her in shock. Courtney hadn’t budged. Caitlin couldn’t tell where Courtney was standing.
Downing’s unease turned into panic. When her husband got home late that night, she told him that, without a doubt, something was wrong with their daughter. They took Caitlin to the pediatrician first thing on Friday morning. The pediatrician sent them to the Arnold Palmer Children’s Hospital in Orlando, Fla., where Caitlin got an MRI scan. The Downings got the DIPG diagnosis on Friday, January 13, 2012.
Caitlin’s symptoms were typical. Parents of DIPG patients report uncoordinated eye movement, uncharacteristic clumsiness, inexplicable mood swings and muscle weakness. The change from a healthy, vibrant child to one stricken with these symptoms happens in a matter of days.
“They progressively lose their ability to move, swallow, smile, talk,” says pediatric neuro-oncologist Michelle Monje at Stanford University. “But kids stay very cognitively alert throughout the course of the disease. They are aware of everything happening to them.”
She pauses. Then she says, with a catch in her voice, “It’s awful.”
Downing, a certified bereavement counselor, says, “There is nothing that can prepare you for when a doctor sits across from you and says, ‘Your child’s got a brain tumor.’ Then the second blow comes when I ask, ‘How do we treat that?’ She says, ‘There is no treatment, Mrs. Downing. You have a maximum of 15 months. We are going to help you enjoy your child.’”
Deciding to donate

The decision to allow a child’s tissues to be used for research stirs up conflicting emotions. Kristine Wetzel and her husband, Dave, decided to donate their daughter McKenna’s brain and spinal cord when she died from DIPG four years ago. A friend and neighbor of the Wetzels, Lisa Roberts, was the one to suggest tissue donation.
Wetzel says the decision to donate came easily as McKenna reached her final hours on July 21, 2011, two weeks shy of her eighth birthday. “It was our way of fighting back,” says Wetzel. “I wanted her life to have meaning, and I wanted her death to have meaning as well.”
When McKenna died, Roberts made the arrangements to have McKenna’s organs sent to Michelle Monje’s laboratory at Stanford. Since then, the Monje lab has established a cell line from her tumor.
But the decision brought up pain. Wetzel, a high-school history and English teacher, says that the first time she held a Petri dish of DIPG cells in the Monje lab was the worst moment of her life after losing McKenna. All she could think was, “This is what is left of my daughter – the thing that killed her is the only thing that is still alive.”
But with the discoveries coming out of the DIPG field, Wetzel has come to see the power of tissue donation. She also finds comfort in the idea that even in a laboratory setting, McKenna lives on as the name of the cell line.
No progress
In DIPG, the cancerous tissue is rooted so intricately into the bed of healthy tissue that it’s impossible to know how far the tumor spreads and where it ends. Surgeons have attempted to biopsy and cut out the tumor, but because of its vise-like grip on healthy tissue, they never have been able to remove the tumor safely.
With the advent of MRI scanners in the 1980s, radiologists found they could diagnose the tumor noninvasively. “There was a kind of moratorium, where it felt inappropriate to biopsy the tumors,” explains pediatric neuro-oncologist Mark Kieran of Dana–Farber/Boston Children’s Cancer and Blood Disorders Center. “The only thing worse than dying of DIPG was to be damaged by the biopsy before you got to die of the DIPG.”
Without biopsies, there weren’t any DIPG tissue samples to study. Clinicians tried to make do with another cancer, adult glioblastoma multiforme. That brain tumor strikes adults and is thought to be similar to DIPG because the two tumors seem to resemble each other under an optical microscope. Any clinical trial to test potential therapies for DIPG was based on what was known about adult glioblastoma. “We’ve done that now for 30 to 40 years and unfortunately made no progress in the disease whatsoever,” states Kieran.
All doctors can offer to DIPG patients is radiation treatment. Downing and another mother of a DIPG patient, Kristine Wetzel, both mention Neil Armstrong’s family. The first man to walk on the moon had a daughter with DIPG. Karen Armstrong died when she was 3 years old in January 1962. Her treatment of cobalt-based radiation wasn’t drastically different from the radiation treatment offered to children with DIPG today. In James R. Hansen’s authorized biography of the astronaut, “First Man: The Life of Neil A. Armstrong,” sources speculate that Armstrong channeled his profound grief over his daughter’s death into becoming an astronaut.
Radiation temporarily shrinks the tumor and gives the children a temporary reprieve, known as the honeymoon period, when they can resume their normal activities. But the tumor comes back within months, more aggressive than before, after which there is nothing left to be done.
Most unexpected finding
In 2007, a group of French neurosurgeons safely performed biopsies of 24 children with DIPG. Kieran, who had been arguing unsuccessfully for the need to biopsy DIPG patients to advance the field, says the French demonstration provided perfect leverage: “We had to argue that either the French neurosurgeons were infinitely more capable than American neurosurgeons or it was time to allow us to start to move forward.”
Getting actual DIPG samples was a major step forward for the field. Another was that the parents of patients with pediatric brain tumors were growing increasingly vocal in advocating for more research and allowing their children to be autopsied.
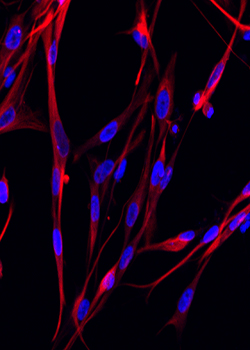 Fluorescent images of cells from a DIPG sample.Image provided by Michelle Monje
Fluorescent images of cells from a DIPG sample.Image provided by Michelle Monje
Indeed, it was with autopsy samples that researchers took an important step in understanding the molecular biology of pediatric brain tumors. In 2012, two groups of researchers, one led by pediatric oncologist Nada Jabado at McGill University and the other by developmental neurobiologist Suzanne Baker at St. Jude’s Children’s Hospital, published data from autopsied pediatric brain tumors. Independently, the groups had sequenced the DNA extracted from the tumors, including DIPG, and discovered mutations in histones.
In the cell’s nucleus, histone proteins form a series of complexes around which DNA wraps. Histone complexes undergo a slew of posttranslational modifications — methylation, acetylation, ubiquitination — to dictate how tightly or loosely DNA wraps around them. The complicated pattern of posttranslational modifications influences whether or not genes around the histones are transcribed.
Four types of proteins make up a histone complex. One is called histone H3. It is one of the most conserved and fundamental components of the histone complexes, and it has three variants, H3.1, H3.2 and H3.3.
In the non-DIPG tumors in the brain’s cerebral cortex, investigators found a mutation in H3.1 that changed the code for a glycine residue called G34, into the code for a valine or arginine residue. This mutation had a ripple effect: A lysine two residues down from G34, called K36, became improperly methylated.
In about 80 percent of the DIPG tumors, investigators discovered a different mutation in histones. This mutation took place in the genetic code for a specific lysine residue called K27. The mutation changed the lysine to a methionine. The mutation is known as K27M. Three-quarters of the DIPG tumors with histone gene mutations had the K27M mutation in H3.3; the remaining quarter had the glycine mutation in H3.1.
The genome contains multiple copies of H3.1 and H3.3. But these mutations turned up only once in a single copy of each gene. Other copies of the genes were normal. The genes “are so redundant that it was unexpected a single one of those genes would be a hotspot” for mutations, says Baker.
Not only does K27M prevent methylation, says histone-expert Allis, but it also looks like the mutation poisons the enzymes that add on the methyl groups, causing methylation to go awry across the genome.
A year after the Jadabo and Baker groups published their results, histone mutations were implicated in other pediatric diseases. Researchers found that H3 gene mutations are present in more than 90 percent of two types of pediatric bone cancers.
Histone mutations now are some of the hottest targets in oncologic drug development. Earlier, scientists had targeted enzymes that modified histone proteins, and several phase I clinical trials are under way to test small molecules against those histone-modifying enzymes. But until the mutations were found in DIPG and other pediatric cancers, no one thought to target the histones themselves.
Researchers have found additional mutations that may affect the progress of DIPG. Last year, investigators found mutations in a gene called ACVR1 that accompany the mutations in H3.1. Unlike the histone-gene mutations, the ACVR1 gene has been implicated in fibrodysplasia ossificans progressiva, another rare disease. In that disease, patients slowly accumulate abnormal bony tissue; the illness sometimes is referred to as ‘stone man disease.’ Connective tissue starts to turn to bone, and patients soon can’t breathe.
While these breakthroughs are exciting, they have left researchers with many questions. How can a mutation in a single copy of a histone gene be so potent as to cause cancer? How do these mutations promote tumor growth? And why do these mutations cause only pediatric forms of brain and bone tumors? There are hints that the spatiotemporal expression of these genes explains why DIPG happens in children only in a certain time frame.
What is obvious, says Becher, is that pediatric cancers are distinctly different from adult cancers. Pediatric cancers are products of developmental pathways gone awry. Adult cancers are manifestations of misregulation of the cell cycle.
The fresh research into what causes pediatric brain cancers is exactly what Keith Desserich, a founder of The Cure Starts Now, wants to see. His 6-year-old daughter Elena, whom Desserich describes as a perfect little lady, died from DIPG in 2007. In Desserich’s view, cancer research has been far too dependent on the trifecta of surgery, radiation and standard chemotherapy.
“For the last 80 years, we focused on the same three tenets and tweaked our way to treatments,” says Desserich. “You can’t make monumental advances through incremental science. You need to change the way you think about everything if you’re going to try to find a cure.”
No more double blows
Researchers are unanimous when they say that the parents of patients have changed the landscape of DIPG research in the past decade by allowing biopsies and autopsies of their children and raising money for research (see “Deciding to donate”). With these resources in hand, researchers are developing appropriate cell lines and animal models that more accurately reflect DIPG as well as carrying out better-informed clinical trials. Today, there are 37 ongoing clinical trials targeting DIPG.
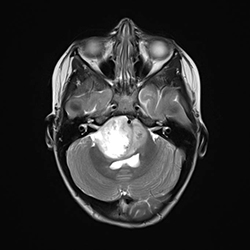 These days DIPG is diagnosed by an MRI scan. It is the bright white patch in the image.Image provided by Weill Cornell Medical College.
These days DIPG is diagnosed by an MRI scan. It is the bright white patch in the image.Image provided by Weill Cornell Medical College.
Parents of DIPG patients talk about balancing making sure their children live full lives in their last few months and going all-out to fight for more time. The Downings, after spending hours combing through the scientific literature, decided to enroll Caitlin in a clinical trial headed up by Souweidane in New York City.
Caitlin was the first child to be enrolled in the trial, which is still ongoing. It is exploring the targeted delivery of a radioactive monoclonal antibody directly into the tumor through a catheter. The Downings had to make several trips between New York City and Orlando, with Caitlin eager to see her beloved “Dr. Mark.” Two weeks after she underwent surgery to embed the catheter into the tumor, Caitlin celebrated her fifth birthday dressed in a resplendently pink Hello Kitty outfit accented with fake pink hair.
When Caitlin died six months later, the Downings donated Caitlin’s brain and spinal cord to Souweidane’s research group. Denise Downing, like every other parent of a child stricken with DIPG, has become a vocal advocate and supporter of more research: “I hope the outcome of doing the science is that someday, that pediatric neuro-oncologist is going to sit across from a set of parents and she’s going to hand them that first blow. She’s going to say, ‘Your child has a brain tumor.’ Those parents are going to panic,” says Downing. “But she’s not going to deliver the second blow, because she’s not going to say, ‘We don’t know how to treat that.’ Instead, she will say, ‘We know how to cure that.’”
With her voice breaking, Downing says, “I know that the day will come.”
Some foundations dedicated to DIPG research
(Author’s note: This is not a comprehensive list.)
- Fly a Kite Foundation, in memory of Zachary Bernstein, age 11
- Hope for Caroline Foundation, in memory of Caroline Cronk, age 5
- Jeffrey Thomas Hayden Foundation, in memory of Jeffrey Hayden, age 12
- Julian Boivin Courage for Cures Foundation, in memory of Julian Boivin, age 5
- The Cristian Rivera Foundation, in memory of Cristian Rivera, age 6
- The Cure Starts Now, in memory of Elena Desserich, age 6
- The McKenna Claire Foundation, in memory of McKenna Claire Wetzel, age 7
- Reflections of Grace Foundation, in memory of Grace Elizabeth Ekis, age 6
- Children’s Brain Tumor Family Foundation, a consortium of families brought together by pediatric brain tumors
Experts discuss DIPG research
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

