From the journals: JLR
What can you do with artificial lipoproteins? A new key to angiogenesis. Flavonoids counteract oxidative stress. Read about papers on these topics recently published in the Journal of Lipid Research.
What can you do with artificial lipoproteins?
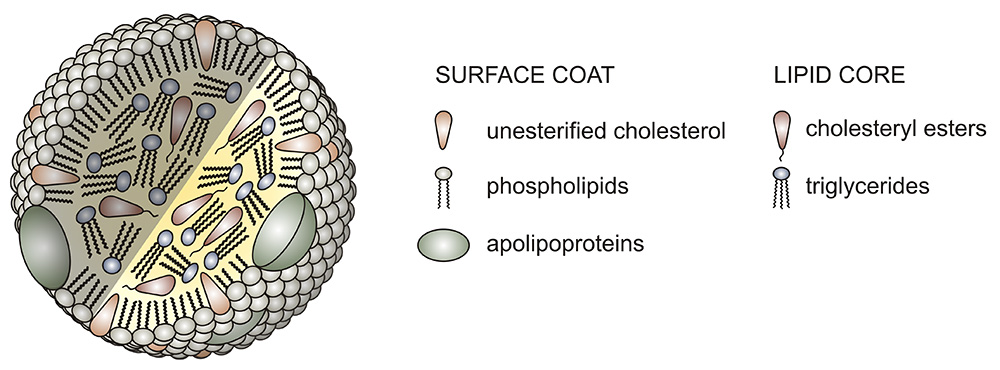
Adiposomes are engineered lipid nanoparticles comprising a neutral lipid core surrounded by a monolayer phospholipid membrane. In a recent study in the Journal of Lipid Research, Zen Cao at the University of the Chinese Academy of Sciences and collaborators detailed how they used adiposomes to generate artificial lipoproteins, or ALPs, novel nanoparticles that mimic naturally occurring lipoproteins. They then characterized ALP functions in biological systems, validating their use as tools for research and medicine.
The authors first explored the feasibility of generating ALPs and characterized how they compare with naturally occurring lipoproteins. Using transmission electron and confocal fluorescence microscopy, they showed that cells take up ALPs via clathrin-mediated endocytosis and the ALPs degrade like native lipoproteins. After confirming ALP viability in culture, the team moved to a mouse model; they injected fluorescently labeled ALPs into the tail veins of mice and looked at where the ALPs ended up in the body. In the heart, lung, kidneys and skeletal muscle, ALPs were metabolized like native lipoproteins. ApoE-coated ALPs have a long lifespan and promote degradation of the low-density lipoprotein receptor. The authors noted that the ALPs displayed physiological functions that were similar to naturally existing lipoproteins, such as improving glucose tolerance in mice and preventing apolipoprotein degradation when the ALPs were synthesized and exposed to trypsin. These results suggest that ALPs could be a new model for studying lipoprotein function because researchers can precisely generate them with specific lipids and apolipoproteins.
In addition, ALPs could be a useful drug delivery system with targeting molecules. While agents such as liposomes have aqueous cores, the ALPs’ hydrophobic core is ideal for housing hydrophobic drugs. Clinicians could use ALP carriers to deliver drugs to tumors since rapidly proliferating cancer cells require the uptake of fatty acids. The clathrin-mediated endocytosis pathway that the authors identified for ALPs would ensure reliable drug uptake, whereas the cellular uptake of other nanoparticle vehicles is inconsistent.
A new key to angiogenesis
Angiogenesis, the development of new blood vessels from existing ones, occurs in two primary forms: physiological (during the development and repair of tissues) and pathological (in diseased tissues). Researchers understand many of the factors that facilitate angiogenesis but have yet to determine the roles of various prostaglandin, or PGD, synthases in driving angiogenesis.
A recent paper in the Journal of Lipid Research by Daiki Horikami and Erika Sekihachi at the University of Tokyo and collaborators described their study of how lipocalin-type PGD synthase, or L-PGDS, and hematopoietic PGD synthase, or H-PGDS, contribute to physiological and pathological angiogenesis. The authors used mice genetically engineered to be deficient for either enzyme to study retinal development. They showed that L-PGDS is expressed in endothelial and neuronal cells, and deficiency reduced physiological angiogenesis during mouse retinal development. H-PGDS is expressed in hematopoietic cells, and deficiency caused increased retinal inflammation.
The ability of H-PGDS to limit inflammation might be leveraged as a therapeutic for diabetic retinopathy, the abnormal growth of blood vessels in the retina. Future research is needed to establish the roles of L-PDGS and H-PDGS in diseases that affect other organs.
Flavonoids counteract oxidative stress
Eukaryotic plasma membranes contain phospholipids that create a barrier separating the intracellular and extracellular spaces. Most membrane lipids contain a polar head group and two fatty acid tails. Many of these tails are polyunsaturated and can be oxidized. This can lead to oxidative stress within membranes, cause cellular toxin buildup and alter signal transduction pathways. Oxidative stress is a factor in various diseases, including neurodegenerative, gastrointestinal, kidney and liver disorders.
A recent study in the Journal of Lipid Research by Anja Sadžak at the Ruđer Bošković Institute in Croatia and colleagues described how the researchers induced membrane defects using dodecanedioic acid, or DDA, and how flavonoids, metabolites from plants, can relieve these defects. Using a liposome model to replicate cell membrane conditions and the DDA treatment to mimic the effects of oxidative stress, the team found that DDA membrane aggregates cause pores to form. Using molecular dynamics simulations and experimental techniques, they showed that adding flavonoids to liposomes under DDA-induced oxidative stress disrupted the DDA aggregates and reversed membrane damage. Flavonoids are found in berries, citrus fruit, tea and other foods. This study highlights their potential to counteract oxidative stress and restore membrane function.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

