Molecular sensor enables water bear hardiness by triggering dormancy
Tardigrades – hardy, microscopic animals commonly known as “water bears” – use a molecular sensor that detects harmful conditions in their environment, telling them when to go dormant and when to resume normal life. A team led by Derrick R. J. Kolling of Marshall University and Leslie M. Hicks of the University of North Carolina at Chapel Hill report these findings in a new study published January 17 in the open-access journal PLoS ONE.
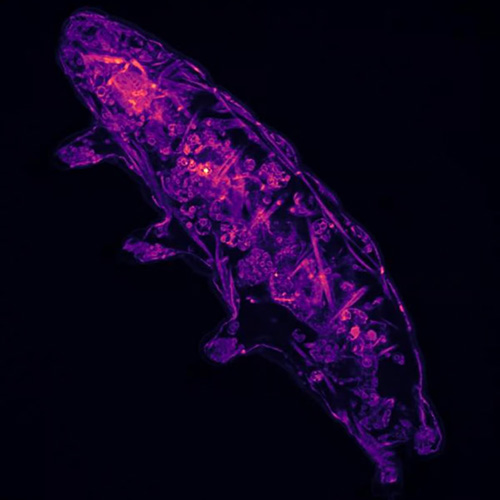
Water bears are famous for their ability to withstand extreme conditions, and can survive freezing, radiation, and environments without oxygen or water. They persist by going dormant and entering a tun state, in which their bodies become dehydrated, their eight legs retract and their metabolism slows to almost undetectable levels. Previously, little was known about what signals water bears to enter and leave this state.
In the new study, researchers exposed water bears to freezing temperatures or high levels of hydrogen peroxide, salt or sugar to trigger dormancy. In response to these harmful conditions, the animals’ cells produced damaging oxygen free radicals. The researchers found that water bears use a molecular sensor—based on the amino acid cysteine—which signals the animals to enter the tun state when it is oxidized by oxygen free radicals. Once conditions improve and the free radicals disappear, the sensor is no longer oxidized, and the water bears emerge from dormancy. When the researchers applied chemicals that block cysteine, the water bears could not detect the free radicals and failed to go dormant.
Altogether, the new results indicate that cysteine is a key sensor for turning dormancy on and off in response to multiple stressors, including freezing temperatures, toxins and concentrated levels of salt or other compounds in the environment. The findings suggest that cysteine oxidation is a vital regulatory mechanism that contributes to water bears’ remarkable hardiness and helps them survive in ever-changing environments.
"Our work reveals that tardigrade survival to stress conditions is dependent on reversible cysteine oxidation, through which reactive oxygen species serve as a sensor to enable tardigrades to respond to external changes," the authors stated.Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

