Life’s little oscillations
Living things must deal with a universe that is both regular and ever-changing: No day exactly mirrors the last, yet the sun and moon still appear at their appointed hours.
Cells contain their own seeming chaos, with countless molecules cooperating to produce subtle responses and behaviors. And in recent decades, a great deal of focus has specifically centered on the periodic patterns that underlie many cellular processes.
Oscillations — such as a pendulum’s swing or a ball’s bouncing on the end of a spring — are among the simplest and most common phenomena in physics, but researchers have come to appreciate their ubiquity in the biological world, too. Concentrations of molecules rise and fall, genes alternate between on and off, and circadian clocks keep time almost as well as human-made machinery. Together, these biochemical fluctuations are crucial for a blizzard of biological needs: timing daily activities, orchestrating cell division and movement, even mapping out parts of an embryo as it grows. Cells would be unable to function without them.
Such patterns were harder to spot in years past because scientists analyzed whole populations of cells at a time and looked at averages, says synthetic and systems biologist Michael Elowitz of Caltech in Pasadena. But biochemists can now tag molecules in individual cells with fluorescent biomarkers and film their ebbs and flows. “More and more people started to look at individual cells over time and discovered that some of the most important systems in biology are not static — they’re really dynamic,” Elowitz says.
Some biochemical oscillations are simple: A few proteins or other organic chemicals go through a repeating pattern. Others are so complex that scientists have yet to map out their pathways. But their pervasiveness has drawn a great deal of attention from those seeking insight into biochemical behavior and researchers like Elowitz who hope to apply such knowledge by engineering novel functions into cells.
“All of these are self-organized,” says theoretical physicist Karsten Kruse of the University of Geneva in Switzerland, who coauthored an article about oscillations in the Annual Review of Condensed Matter Physics. “If you add the right components together, then they don’t have a choice — they must produce these oscillations.”
Here’s a look at some of the most well-studied and intriguing biochemical oscillations that emerge from the complexity of the cell to produce order.
Circadian rhythms in cyanobacteria
Daily activity cycles are important for survival in our 24-hour world. In 2017, the Nobel Prize in Physiology or Medicine went to researchers who unraveled the details underlying these rhythms in higher creatures. In contrast, single-celled organisms, such as light-harvesting blue-green algae or cyanobacteria, were once thought too simple and fast-dividing to harbor such clocks.
But keeping track of the sun is obviously important for organisms whose livelihood depends on light. Today researchers know that these life forms also have intrinsic circadian rhythms — and know a lot about how they function. Molecular geneticist Susan Golden of the University of California, San Diego, has helped to decode the molecular machinery regulating time in the cyanobacterium Synechococcus elongatus, and coauthored a description of the clock in the Annual Review of Genetics. The story goes like this:
The cyanobacterial circadian rhythm relies on an oscillation among three proteins: the enormous KaiC, which consists of two six-sided, doughnut-like rings stacked atop one another; its helper, the butterfly-shaped KaiA; and the component KaiB, which is usually inert but can spontaneously change to a rare, active form.
As the sun rises, wiggly molecular chains extending from the top of KaiC’s upper stack grab hold of little KaiA. Once bound, KaiA induces the immense KaiC to accept phosphate groups. Over the course of the day, more and more phosphate is added to KaiC’s top ring, stiffening it and causing its lower doughnut to deform.
By sunset, the lower ring has been so squished that it exposes a hidden binding site along its bottom. The rare active form of KaiB can now stick to this site, changing KaiC’s structure so it lets go of KaiA. As the night progresses, KaiC slowly gives up phosphates, eventually returning to its original state and releasing KaiB. The cycle takes about 24 hours.
And how does this oscillation cause rhythms in the cell’s biochemical activities? By cyclically activating a key gene-regulating protein named RpaA. RpaA switches on (or off) around 100 genes in S. elongatus. These genes, in turn, direct the cell’s metabolism and physiology — telling it, for instance, when it’s time to photosynthesize or burn sugar stores. Since RpaA activity peaks at dusk, the bevy of activities occur with daily cycles.<*
Division in E. coli
Bacteria divide to reproduce, but an off-center partition will cause lopsided daughter cells, potentially leaving descendants understocked with the materials they need to survive. Not surprisingly, then, many microbes use molecular systems to split perfectly in half.
Perhaps the best understood is a team of three globule-shaped proteins called MinC, MinD and MinE that create waves of fluctuations in Escherichia coli.
The key component is MinC — in high concentrations, it blocks a protein that kicks off the process of division. But MinC doesn’t work solo. On its own, it will diffuse throughout an E. coli cell and stop division from happening anywhere at all. So MinC relies on MinD and MinE to tell it where to go.
MinD binds to the membrane at one end of the cell, painting the interior with clusters of itself. That attracts huge collections of MinC that come in and bind to MinD — blocking the molecular machinery that initiates division from setting up shop at that location.
Next comes the work of MinE. Lots of MinEs are attracted to the MinDs and they force MinD to undergo a small change. The result: MinDs and MinCs are kicked off the membrane. They move on to search for a place devoid of MinEs — like the other side of the bacterium — where they can bind once again to the cell membrane.
Then it happens all over: MinEs chase and kick off the MinD-MinC complexes again. Wherever MinD tries to stick to the wall, it gets booted out, and MinC along with it. The process generates a pulsation of Min proteins that moves back and forth between the cellular antipodes over the course of a minute.
Why does this cause the cell to divide right in the center? Because MinC spends the least time in the middle of the cell — giving the division machinery an opportunity to assemble there.
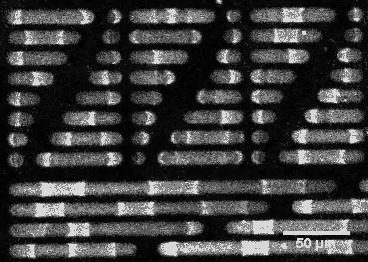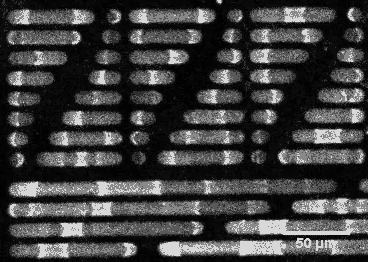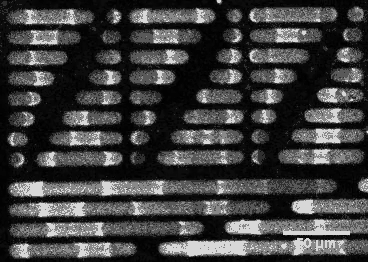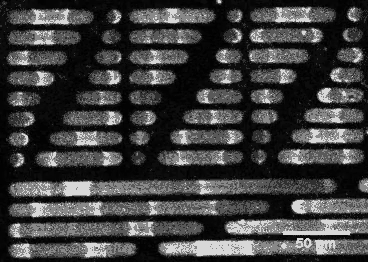
This wouldn’t be the case if E. coli’s sizing were different. By constructing synthetic rod-shaped compartments of different lengths and widths and introducing concentrations of MinD and MinE into them, biophysicist Petra Schwille of the Max Planck Institute of Biochemistry in Munich, Germany, and colleagues created beautiful videos of the molecules’ fluctuations. They showed that longer or shorter cells would allow the division site to be at other locations.
Vertebrate segmentation
In the seventeenth century, Italian physiologist Marcello Malpighi used an early microscope to study developing chicken embryos and observe the formation of their spinal columns. More than 300 years later, modern researchers are still puzzling over the incredibly complex process that forms each vertebra and segment of the body. One key component: a clock-like oscillation that travels down the developing embryo.
“It’s easiest to think about it as an oscillator that gets displaced in space with a certain speed and direction,” says developmental biologist Olivier Pourquié of Harvard Medical School in Boston. Each time the embryo reaches a certain phase in the oscillation, it stamps out a segment. Then it goes through the cycle again, producing a second segment. And so on. “But because the oscillator moves, it will stamp the segment at a different position,” Pourquié says. “In this way, you can generate a sequential series of segments” along the length of a gradually extending body.
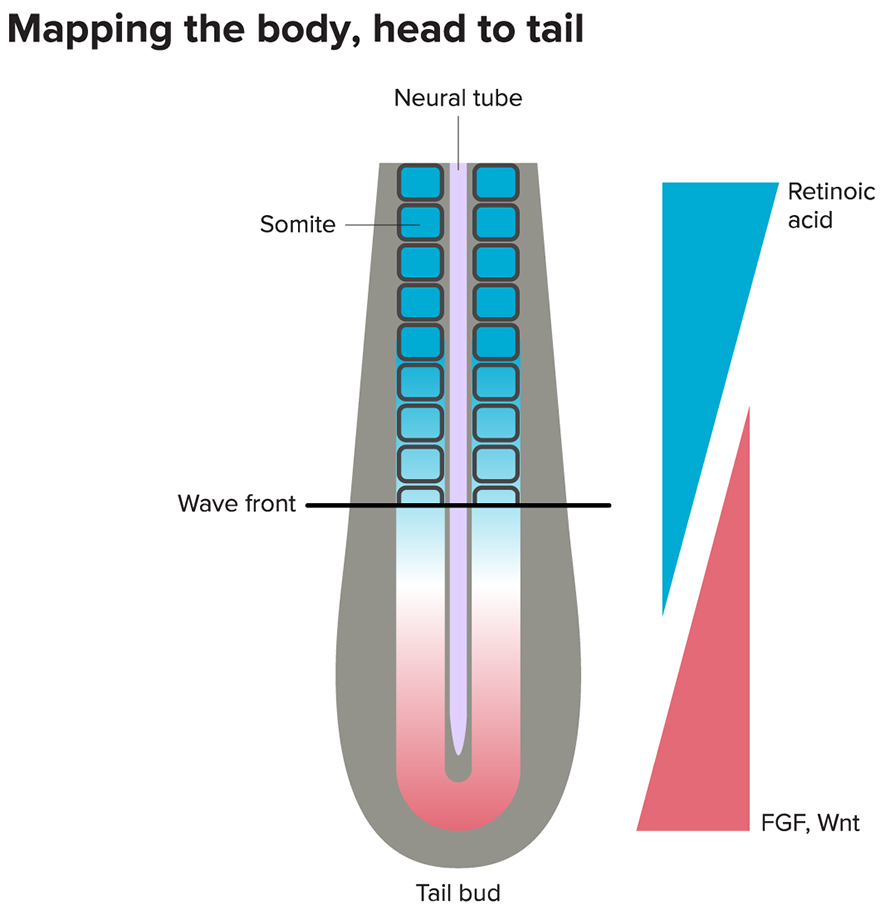
In embryos of vertebrates like fish, chickens, mice and humans, the future head is one of the first structures to appear. Later, bumpy segments called somites emerge, one by one, below the head, eventually giving rise to the spine, rib cage, skeletal muscles, cartilage and skin of the back. These ball-like pairs of somites are generated from tissue below the head when that tissue receives cues from two separate systems — called the wavefront and the clock — at the same time.
First, the wavefront. It involves two molecules, fibroblast growth factor (FGF) and Wnt, each of which forms a gradient, with their highest levels farthest from the head: a place near the tail that is constantly moving away as the embryo elongates. (An inhibitory substance called retinoic acid, produced by already formed somites, helps to keep FGF-Wnt activity toward the rear.) The two molecules set off a complex series of steps and act to inhibit somite formation. Somites appear right around the spots where they are least abundant.
Second, the clock component. That’s governed by a third molecule — called Notch — and the signaling pathway it sets off. Notch causes cells to oscillate between active, “permissive” states and inactive, “restrictive” states at a characteristic rate that varies from species to species. If the cells happen to be in a permissive state at a spot where the Wnt-FGF gradient has sufficiently weakened, a cascade of genetic activity tells cells in that region to gather into somites.
And as the body elongates and the tail moves farther from the head, the Wnt-FGF wavefront will move in a posterior direction, stamping out a line of somite segments with each tick of the Notch clock. (Read more about segment formation in this article in Knowable Magazine’s special report on Building Bodies.)
Waving motion
Just like their multicellular kin, single-celled creatures need to move in order to hunt, escape predators or seek out light and nutrients. But getting around when you don’t have limbs can be a tough task. So cells that need to move, be they free-living or part of a multicelled creature, rely on various types of molecules to do the job. In certain cases, the action of these molecules can induce wave-like ripples on the cell’s surface, which the cell uses to skate forward.
Actin, a protein found broadly in nature, is key. The molecule, a major component of the mesh-like cytoskeleton, is involved in a slew of operations: mobility, contraction as cells divide, changes in cell shape and internal transport.
Along with colleagues, computational biologist Alex Mogilner of New York University in New York City has investigated how actin can drive waves that allow certain types of fish cells known as keratocytes to crawl around. Keratocytes are responsible for producing collagen and other connective proteins, moving to sites of injury and inflammation to assist in healing. They have often been used as model systems to study cell locomotion.
Normally, cells get around by protruding long, limb-like extensions and tottering forward like tiny, exotic aliens. But when they enter an especially sticky environment, their strategy changes and they no longer extend thin limbs, instead skimming forward using short ruffling motions of their cell membranes.
Beneath the membrane of a keratocyte, actin proteins are constantly assembling and disassembling into long filaments. In a highly adhesive environment, the cell membrane will sometimes stick to the external material, which tugs on the membrane as the cell tries to move. This tugging creates a small pocket right beneath the membrane that actin filaments can expand into.
An enzyme called vasodilator-stimulated phosphoprotein (VASP) will often be hanging around beneath the membrane, too. VASP binds to the actin and stimulates it to form even longer filaments and branches. If both VASP and actin are present in high enough concentrations, a cascade of actin filament-lengthening can begin. “When it starts, it’s like a fire starting,” says Mogilner.
The elongating filaments push on the tight cell membrane, producing a bump that gives the actin chains room to grow even more, and bind more VASP. Tension in the membrane causes it to sway like an audience doing “the wave,” sending the cell skating in the wave’s direction. The actin filaments beneath the membrane grow sideways as well as forward, helping to push the wave along. At the original spot where the wave began, the actin filaments will have used up all the available VASP, preventing further lengthening. The sticky external environment adhering to the taut membrane also dampens the wave at the origin spot.
“In a way, VASP proteins are like trees, actin filaments are like fire, and adhesions and membrane are like water: At the back of the wave, trees are all burnt and drenched in water, and the fire stops,” says Mogilner. But at parts of the membrane far from the wave’s origin, high concentrations of actin and free VASP will still exist, often leading to a new wave that begins where the previous one was extinguished.
It’s still unclear just how keratocytes choose what direction to move in. Presumably, says Mogilner, the leading edge of a cell is oriented toward some external cue, like a chemical gradient from some food. Also poorly understood are the benefits of this particular mobility tactic. “In some cases, it’s not obvious why waves are better than other mechanisms,” says Kruse, whose work on cytoskeleton dynamics focuses on theoretical descriptions of cell movement and division.
Some researchers have suggested that the wave-like motion might help cells get around small obstacles that they would otherwise run into head-on. Or maybe it’s prudent for them not to overextend their limb-like protrusions in certain environments.
A synthetic cellular circuit
When Caltech’s Elowitz was in graduate school at Princeton University in the 1990s, he often got frustrated by diagrams showing the inferred interactions of genes and proteins, with their many unknowns and arrows going every which way. “I just became convinced that if we really want to understand these things we need to be able to build them ourselves,” he says.
Along with his advisor, Stanislas Leibler, he created a synthetic genetic oscillator in order to show that a simple biological system could be programmed and built from scratch. Called the repressilator, it consists of a tiny loop of DNA with three genes on it. They carry instructions for making three proteins called repressors, each of which binds to the next gene and turns it off.
And here’s where it got fun. In their construction, the first gene produced a repressor protein, LacI, which would shut off the second gene, called tetR, whose product would shut off the third gene, cI, whose product would shut off the first gene.
“It’s like a game of rock, scissors, paper,” says Elowitz. “The first repressor turns off the second one, the second turns off the third one, and the third turns off the first one.” Once the first gene is turned off, the second gene can turn on, and thus turn off the third gene. And then the first gene can turn on again — and on and on.
To watch the circuit run, Elowitz included a fourth gene that would cause E. coli to light up bright green — but only when it was turned on by one of the three repressors. Placed inside E. coli, the repressilator causes the microbe and its descendants to flash green fluorescent light with a period of around 150 minutes.
Beyond simply showing that such circuits could be created, the research provided insight into the noise of biological systems. E. coli did not turn out to be a perfect little deterministic machine, says Elowitz. When loaded with the repressilator, some daughter cells flashed more strongly or weakly than others, suggesting that there is a great deal of variability inherent in their biochemical workings.
Studies have continued on the system and, in 2016, a team at Harvard University and the University of Cambridge significantly improved the precision of the circuit, allowing much larger numbers of daughter cells to flash in sync.
The field of synthetic biology has grown rapidly in the two decades since Elowitz’s early work, and now offers a plethora of interesting applications, including novel proteins and enzymes for medicine, biological sensors and even cells that perform calculations like living computers. Being able to fine-tune biochemical oscillations — with far more exquisite precision than can be found in natural systems — will be crucial to building future synthetic biological products, says Elowitz.
“Out of physics, we have electronics and electrical engineering,” he says. “We’re just beginning to learn these principles of genetic circuit design, and I think we’re at an interesting moment.”
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

