Immune cells become exhausted within hours of encountering tumors
A key function of our immune system is to detect and eliminate foreign pathogens such as bacteria and viruses. Immune cells like T cells do this by distinguishing between different types of proteins within cells, which allows them to detect the presence of infection or disease.
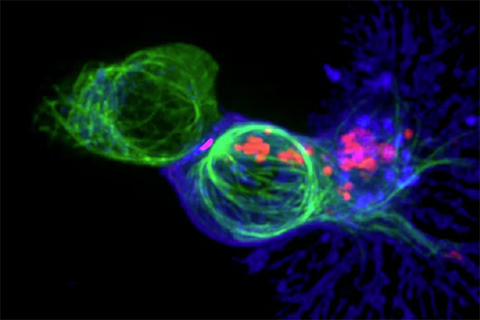
A type of T cell called cytotoxic T cells can recognize the mutated proteins on cancer cells and should therefore be able to kill them. However, in most patients, cancer cells grow unchecked despite the presence of T cells.
The current explanation scientists have as to why T cells fail to eliminate cancer cells is because they become “exhausted.” The idea is that T cells initially function well when they first face off against cancer cells, but gradually lose their ability to kill the cancer cells after repeated encounters.
Cancer immunotherapies such as immune checkpoint inhibitors and CAR-T cell therapy have shown remarkable promise by inducing long-lasting remission in some patients with otherwise incurable cancers. However, these therapies often fail to induce long-term responses in most patients, and T cell exhaustion is a major culprit.
We are researchers who study ways to harness the immune system to treat cancer. Scientists like us have been working to determine the mechanisms controlling how well T cells function against tumors. In our newly published research, we found that T cells become exhausted within hours after encountering cancer cells.
Timing T cell exhaustion
By the time most patients are diagnosed with cancer, their immune system has been interacting with developing cancer cells for months to years. We wanted to go back earlier in time to figure out what happens when T cells first encounter tumor cells.
To do this, we used mice genetically engineered to develop liver cancers as they age, similarly to how liver cancers develop in people. We introduced trackable cytotoxic T cells that specifically recognize liver cancer cells to analyze the T cells’ function and monitor which of the genes are activated or turned off over time.
We also used these same trackable T cells to study their response in mice infected with the bacteria Listeria. In these mice, we found that the T cells were highly functional and eliminated infected cells. By comparing the differences between dysfunctional T cells from tumors and highly functional T cells from infected mice, we can home in on the genes that code for critical proteins that T cells use to regulate their function.
In our previous work, we found that T cells become dysfunctional with dramatically altered genetic structure within five days of encountering cancer cells in mice. We had originally decided to focus on the very earliest time points after T cells encounter cancer cells in mice with liver cancer or metastatic melanoma because we thought there would be fewer genetic changes. That would have allowed us to identify the earliest and most critical regulators of T cell dysfunction.
Instead, we found multiple surprising hallmarks of T cell dysfunction within six to 12 hours after they encountered cancer cells, including thousands of changes in genetic structure and gene expression.
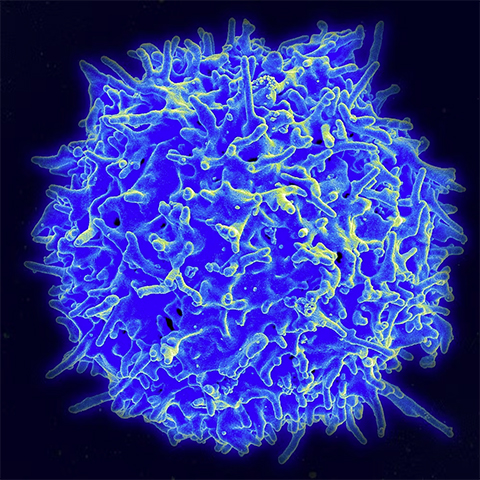
We analyzed the different regulatory genes and pathways in T cells encountering cancer cells compared to those of T cells encountering infected cells. We found that genes associated with inflammation were highly activated in T cells interacting with infected cells but not in T cells interacting with cancer cells.
Next, we looked at how the initial early changes to the genetic structure of T cells evolved over time. We found that very early DNA changes were stabilized and reinforced with continued exposure to cancer cells, effectively “imprinting” dysfunctional gene expression patterns in the T cells. This meant that when the T cells were removed from the tumors after five days and transferred to tumor-free mice, they still remained dysfunctional.
Boosting T cell killing
Altogether, our research suggests that T cells in tumors are not necessarily working hard and getting exhausted. Rather, they are blocked right from the start. This is because the negative signals cancer cells send out to their surrounding environment induce T cell dysfunction, and a lack of positive signals like inflammation results in a failure to kick T cells into high gear.
Our team is now exploring strategies to stimulate inflammatory pathways in T cells encountering cancer cells to make them function as though they are encountering an infection. Our hope is that this will help T cells kill their cancer targets more effectively.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.


