JBC: Molecules from breast milk and seaweed suggest strategies for controlling norovirus
Norovirus is the most common cause of gastroenteritis worldwide; it causes hundreds of thousands of deaths each year and is particularly risky for children under 3 years old. If someone gets norovirus in a setting like a hospital, it’s critically important to protect others from getting infected. Research from universities in Germany, published in the Journal of Biological Chemistry, suggests that it may be easier than anticipated to find a compound that could be used as a food supplement to stop the spread of norovirus in children’s hospitals.
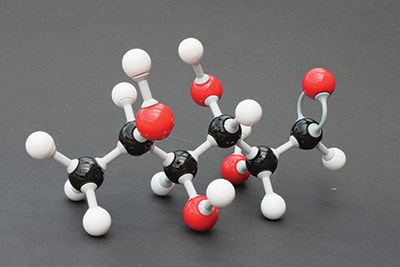 A model of a fucose molecule. Norovirus causes disease after entering cells in the gut by binding to fucose, a sugar molecule found on cell surfaces.Bim in Gartent/Wikimedia Commons
A model of a fucose molecule. Norovirus causes disease after entering cells in the gut by binding to fucose, a sugar molecule found on cell surfaces.Bim in Gartent/Wikimedia Commons
Norovirus causes disease after entering cells in the gut by binding to a sugar molecule called fucose, which is found on cell surfaces. Fucose also is found in breast milk and other foods. Norovirus can’t tell the difference between fucoses that are part of cells in the gut and those that are simply passing through; for this reason, adding a fucose-based supplement to the diet as a decoy could be a way to capture the virus and keep it from infecting cells.
To develop this strategy, however, researchers needed to understand which features of fucose and virus molecules affected how well they attach to each other. In cells, foods and milk, fucose rarely is found as a single molecule; rather, it’s part of chains or networks of sugars and proteins. Franz-Georg Hanisch, a researcher at the University of Cologne, led a project to disentangle these molecular elements and understand what kind of fucose-based product best would distract noroviruses. He started by screening the many types of fucose-containing human milk oligosaccharides, or HMOs.
To Hanisch’s surprise, the strength of the binding between the norovirus protein and HMOs did not depend much on the structure of the HMO or the types of fucose molecules it contained. Rather, what mattered was how many fucoses the HMO contained. Each individual fucose stuck weakly to the virus protein, but the more fucoses there were in the compound, the better the compound and the viral protein stuck together.
“The binding of the virus is not dependent in any way on further structural elements (of HMOs),” Hanisch said. “It’s only the terminal fucose which is recognized, and the more fucose at higher densities is presented, the better is the binding.”
Hanisch turned to the industry standard of where to get a lot of fucoses fast. Brown algae — the family of seaweed that includes kelp — produce a compound called fucoidan, which is a complex network of many fucoses. (Fucoidan has been explored independently as a treatment for HIV and other viruses for unrelated biochemical reasons.)
“There are procedures for isolating the stuff in quite high yields and in high purity,” Hanisch said.
The organization of the fucoses in fucoidans looks nothing like any fucose-containing molecules found in the human body, but fucoidan nevertheless tightly bound to the virus protein in the team’s experiments. This means fucoidan could be a safe and cheap food additive to block viruses from infecting cells. It also suggests researchers will be able to design an even better fucose-containing compound.
Hanisch and his collaborators are moving on to experiments with live viruses and live organisms, with the goal of developing a fucose-based food supplement that could be given to a group of people, such as hospitalized children, at the first sign of a norovirus outbreak, to prevent the circulating viruses from entering their cells and causing disease.
“I hope that in about three years we will have a product which can be used in norovirus defense and to go into clinical studies,” Hanisch said.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Cracking cancer’s code through functional connections
A machine learning–derived protein cofunction network is transforming how scientists understand and uncover relationships between proteins in cancer.

Gaze into the proteomics crystal ball
The 15th International Symposium on Proteomics in the Life Sciences symposium will be held August 17–21 in Cambridge, Massachusetts.
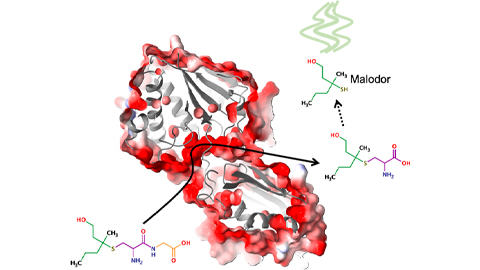
Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.

Neurobiology of stress and substance use
MOSAIC scholar and proud Latino, Bryan Cruz of Scripps Research Institute studies the neurochemical origins of PTSD-related alcohol use using a multidisciplinary approach.
Pesticide disrupts neuronal potentiation
New research reveals how deltamethrin may disrupt brain development by altering the protein cargo of brain-derived extracellular vesicles. Read more about this recent Molecular & Cellular Proteomics article.

A look into the rice glycoproteome
Researchers mapped posttranslational modifications in Oryza sativa, revealing hundreds of alterations tied to key plant processes. Read more about this recent Molecular & Cellular Proteomics paper.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)