Customizing mRNA is easy
While using mRNA as medicine is new, mRNA has been inside you for your entire life. The cells in your body create mRNAs that serve as instructions to make specific proteins you need to function. Researchers can create new mRNAs to correct those instructions when they aren’t working.
I am a molecular biologist who studies how cells control their mRNAs to make the proteins they need, a basic question of how life works at the cellular level. While most scientists studying mRNAs are not creating new drugs, this fundamental understanding of how mRNA works laid the foundation for other scientists to create effective mRNA medicines like COVID-19 vaccines.
By tweaking these instructions, scientists can create powerful new medicines to repair a variety of problems in your cells.
What does mRNA do?
To understand what the mRNAs in your cells are doing for you, let’s start with its more well-known relative, DNA.
DNA is like a set of cookbooks full of different recipes, or genes, to make proteins. People make about 100,000 different proteins that are essential for normal function, such as breaking down nutrients and carrying out other important chemical reactions.
When cells need to make one of those proteins, they don’t read the recipe directly from DNA. Instead, they make a copy in the form of a similar molecule – that’s the mRNA. The “m” stands for messenger, as mRNA contains the message, or recipe, that codes for a protein. About one-third of a cell’s energy is devoted to maintaining the proteins you need, so cells are well equipped to recognize, use and then destroy mRNA once it’s no longer needed.
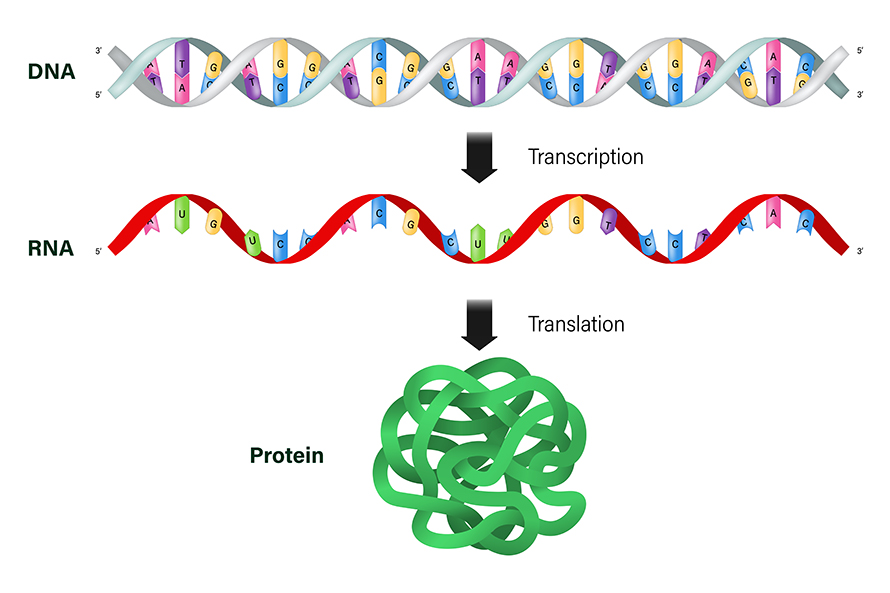
The language of mRNA is made of four building blocks called nucleotides, nicknamed A, U, C and G. The recipe to make a protein contains only three-letter words, meaning there are just 64 possible words. Scientists know exactly which words correspond to each protein building block, so they can easily read an mRNA recipe and know what protein will be made. Mutations in the DNA cookbook can alter or delete an mRNA recipe, leading to disease-causing mistakes in critical proteins.
Why do mRNAs make great medicine?
While mRNA has been within us all along, it took decades of research for scientists to understand how cells recognize mRNA and use it to make protein. But it eventually became clear that mRNA could be a powerful medical tool.
Since scientists understand how mRNAs code for proteins, they can easily create recipes for any protein. These recipes can be edited to meet the needs of the patient, whether this means providing a whole new mRNA recipe or tweaking an existing one to make a slight variation of the protein.
Producing mRNA treatments is also scalable because scientists can make large amounts of mRNA in the lab. The method to make one mRNA is the same for all mRNAs, unlike typical drugs where each compound has its own unique chemistry and requires different manufacturing methods. It’s like learning how to make risotto: Once you’ve learned the basic recipe, you can make endless variations.
Another benefit of using mRNAs as drugs are cells’ natural ability to destroy them when they aren’t needed. Since mRNAs aren’t permanent, doses can be easily changed to meet the changing needs of the patient.
mRNA vaccines beyond COVID-19
The COVID-19 vaccines from Moderna and Pfizer-BioNTech are the first mRNA-based medicines to gain FDA approval. When these vaccines are injected into your arm, the mRNA is absorbed into some of your cells, which read the mRNA recipe and make the spike protein the virus uses to invade cells. Your immune system recognizes this spike protein as foreign and makes antibodies that prepare your body to attack the virus if you encounter it later.
These mRNA vaccines demonstrate the flexibility of mRNA-based therapies. As the virus that causes COVID-19 mutates, new viral variants can evade existing antibodies and cause new waves of illness. However, scientists are able to sequence new mRNA recipes based on these variants and tweak the vaccine recipes to match them. Boosters containing these edited recipes teach your body to make new antibodies that target the latest versions of the viral spike protein.
There are already clinical trials underway for other mRNA-based vaccines, including vaccines for seasonal flu, herpes and respiratory syncytial virus.
There are also many more vaccines in earlier stages of development to combat diseases like norovirus, Lyme disease, Zika and shingles.
mRNA as treatment for disease
The potential for mRNA-based medicine extends beyond vaccines to prevent infectious disease. One example is the use of mRNA to treat cancer.
Some mRNA cancer treatments work like vaccines by training your immune system to specifically target cancer cells. As cancer cells grow, they rapidly gain mutations in many genes. Cancer vaccines contain mRNA recipes based on mutations commonly found in certain types of tumors. When injected into the body, the mRNAs from the vaccines allow normal cells to make those mutated proteins and broadcast them to the immune system, ramping up production of antibodies. These antibodies bind to cancer cells and mark them for immune attack.
Finding the correct protein target for a given cancer is essential. Ideally, the target is unique to the cancer cell so the immune system doesn’t attack healthy cells. The target protein should also be easy for the immune system to sense, making surface proteins good targets. Cancer vaccines, like BioNTech’s BNT-111 for melanoma, target the most common cancer mutations in hope of helping many patients. But patients won’t benefit from the treatment if their cancer cells don’t have those particular mutations.
Because it is so easy to change the mRNA recipes, cancer vaccines can be part of a personalized medicine plan where doctors sample a patient’s tumor, sequence key genes and adjust the mRNA treatment to include recipes specific to that patient’s cancer. Clinical trials using this personalized approach for pancreatic cancer are underway.
The future of mRNA-based medicine
Many diseases arise from cells making the wrong protein, a mutant version of protein or too little of the normal protein. If scientists can deliver a corrected version of the mRNA recipe to enough affected cells, then the mRNA will provide the means to make the proper protein.
Scientists are exploring the use of mRNA to treat heart disease, neurodegenerative disease, bone loss and much more. Although most of these studies are still very early in development, they provide hope for future treatments using mRNA for protein replacement therapies.
For example, one mRNA drug increases the formation of new blood vessels, which can improve wound healing in diabetic patients who have poor blood circulation and higher amputation risks. Another example is using mRNAs to treat propionic acidemia, a disease where children have low levels of two liver proteins that normally prevent toxic byproducts from building up in the body.
The ability to easily customize and produce mRNA increases their potential as effective, personalized therapies – with fewer side effects – that can help many people.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

