JBC: On the trail of steroid aromatase
 Kenneth J. Ryan
Kenneth J. Ryan
Male and female animals typically display numerous differences. However, the two major hormone classes responsible for these differences, androgens and estrogens, differ only subtly in their chemical backbones: Androgens have a six-carbon nonaromatic ring — the A ring — in their steroid skeleton, whereas estrogens have an aromatic A ring.
A single protein, steroid aromatase (also called estrogen synthase), is the only known enzyme capable of aromatizing the A ring in androgens to produce estrogens.
Females and males require both sex hormones in a balance appropriate for each sex. Steroid aromatase maintains this balance during development and pregnancy and in reproductive systems and tissues. Researchers have long sought to better understand steroid aromatase activity in order to address complications during pregnancy, develop hormone-based contraceptives and manage estrogen-responsive cancers.
Three papers published in the Journal of Biological Chemistry authored by Kenneth J. Ryan and now recognized as classics laid the groundwork for understanding the role of steroid aromatase and other steroid-modifying enzymes in estrogen biosynthesis.
Before he embarked on his studies of placental steroid aromatase, Ryan, in one of these JBC papers, reported that microsomal fractions of adrenal glands from beef contain an enzyme activity that hydroxylates carbon 21 in the hormone progesterone and several of its steroid derivatives. This finding clarified the enzymatic nature of this pivotal step in steroid hormone production in adrenal tissues and helped establish an experimental system that Ryan then used to probe how estrogens are made from androgens.
In the 1950s, studies using extracts from animal tissues hinted at the enzymatic nature of the androgen-to-estrogen conversion. However, the amounts of enzyme activities in these preparations were insufficient for detailed investigations to improve understanding of the enzymes and mechanisms in these reactions. This prompted Ryan, then a clinician-researcher at Harvard Medical School, to try to boost estrogen production, extract these hormones more efficiently and begin to characterize the enzymes involved.
“He was one of the first people who did that sort of stuff,” said Richard Auchus, a researcher at the University of Michigan with a long-standing interest in steroid production and modification.
Ryan was an obstetrician and had a ready supply of tissue ideal for investigating estrogen production — human placentas obtained immediately after delivery.
Preparing and analyzing these biological materials was laborious. Ryan extracted the protein activities and products from kilograms of placental tissues, and even small glitches in the experimental protocols could result in loss of activity.
This effort bore fruit when Ryan confirmed in 1959 that the formation of the aromatic A ring is the result of enzymatic activity leading to aromatization. “He figured out that (the enzyme activity) was in the microsomal fraction and that it required NADPH (the reduced form of nicotinamide adenine dinucleotide phosphate), and he developed the chromatography to measure it,” Auchus said.
Ryan’s findings suggested that the aromatization involved an enzyme system that used molecular oxygen to achieve A-ring aromatization, but with the tools available at the time, Ryan could not delineate the exact biochemical sequence of events.
Initially, it wasn’t clear whether the aromatization was performed by one enzyme or by several, because the reaction sequence required at least three oxidations, which yielded some, at the time, unusual products, Auchus said.
It “was fascinating that you got an aromatic A ring,” Auchus said, but “this didn’t really make a lot of sense to people back then. The reaction was weird because it involved both loss of a methyl group and formation of the aromatic A ring.”
The reaction mechanisms remained black boxes, but Ryan’s work had begun to pry the lids open, Auchus said. “Now people could start to look at the mechanism.”
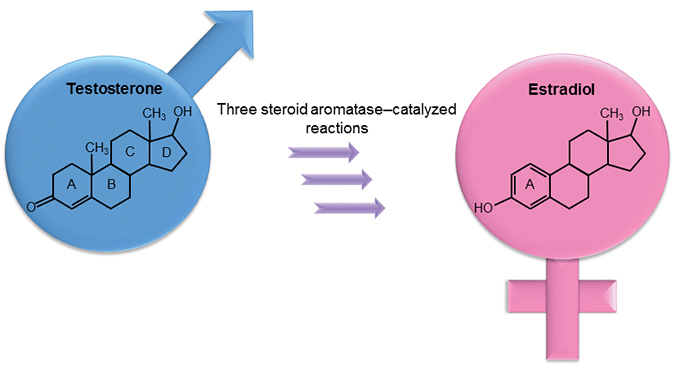 Steroid aromatase, or estrogen synthase, is a cytochrome P450 enzyme that aromatizes the A ring in androgens, such as testosterone (typically present at higher levels in men), thereby producing estrogens, such as estradiol (usually present at higher levels in women), in three separate reactions.Martin SpieringNumerous studies followed, elucidating the reaction mechanism, structure and biological roles of steroid aromatase. In the 1980s, several researchers showed that the aromatizing activity is indeed performed by just one enzyme, a cytochrome P450 monooxygenase aptly named steroid aromatase.
Steroid aromatase, or estrogen synthase, is a cytochrome P450 enzyme that aromatizes the A ring in androgens, such as testosterone (typically present at higher levels in men), thereby producing estrogens, such as estradiol (usually present at higher levels in women), in three separate reactions.Martin SpieringNumerous studies followed, elucidating the reaction mechanism, structure and biological roles of steroid aromatase. In the 1980s, several researchers showed that the aromatizing activity is indeed performed by just one enzyme, a cytochrome P450 monooxygenase aptly named steroid aromatase.
Ryan next set his sights on establishing the biochemical origins of another important estrogen, estriol, now a standard biomarker in routine pregnancy care. Scientists knew estriol was produced by a classic pathway involving the hydroxylation of carbon 16 in the estrogens estradiol and estrone, but Ryan had found preliminary evidence for another pathway in which estriol also could be produced by aromatization of C-16 hydroxylated androgens.
Using his placental assay, Ryan confirmed his earlier finding, and he clarified that another estrogen, 16alpha-hydroxyestrone, is an intermediate in the classic estriol-producing pathway. These discoveries underscored the utility of Ryan’s assay and represented key early steps in untangling the biochemical complexities in estrogen production.
Born in 1926 in New York City, Ryan grew up during the Great Depression, working on farms in his teen years and, after graduation from high school, serving in the U.S. Navy during World War II. He enrolled at Northwestern University for his undergraduate studies and then attended Harvard Medical School, graduating magna cum laude in 1952.
During residencies at hospitals in the Boston area, Ryan landed a biochemistry fellowship shared with Nobel laureate Fritz Lipmann, enabling him to pursue his interest in the roles of estrogens in the biology of pregnancy.
In the 1960s, Ryan began taking on administrative duties, successively becoming chairman of several obstetrics and gynecology departments across the country. In the early 1970s, he returned to Harvard, where he helped build an academic OB-GYN department. In addition to his work in the clinic and research activities, Ryan trained and mentored many students and residents.
He also became active in medical ethics. “He actually got involved with a lot of ethical issues, like fetal tissue research,” Auchus said. “In his later years, he became a pretty prominent person in that field.”
Ryan chaired the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. This commission produced the Belmont Report in 1978, whose guidelines for protecting the rights and dignity of human subjects in research continue to provide an ethical framework for research and health providers in the United States to this day. Ryan also was an early and strong proponent of reproductive choice.
He died in 2002 at the age of 75.
This article originally appeared in the Journal of Biological Chemistry as a JBC Classic. It has been edited for ASBMB Today.Click here to read more JBC Classics.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

