From the journals: JLR
We offer summaries of papers recently published in the Journal of Lipid Research.
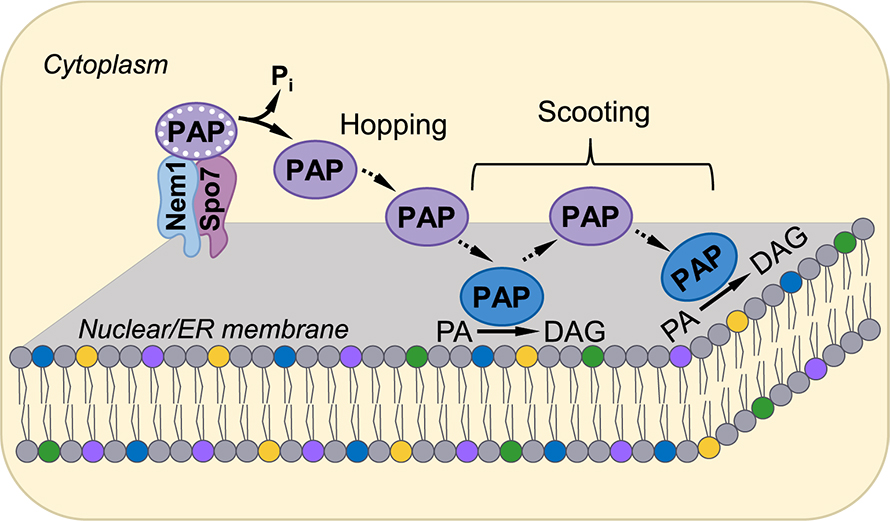
An enzyme hops and scoots on the phospholipid bilayer
Phosphatidic acid phosphatase is an important lipid metabolic enzyme in eukaryotes. It converts phosphatidic acid, or PA, to diacylglycerol, which is needed for the synthesis of the neutral lipid triacylglycerol. The diacylglycerol can also be used for the synthesis of membrane phospholipids.
PA phosphatase lacks a transmembrane domain and is confined in the cytoplasm as an inactive phosphorylated enzyme. Activation occurs when the enzyme is dephosphorylated by a protein phosphatase complex at the nuclear/endoplasmic reticulum, or ER, membrane followed by its interaction with the membrane bilayer and substrate PA. Thus, PA phosphatase requires the membrane surface for catalysis.
To evaluate PA phosphatase activity, researchers have used a system composed of detergent/PA-mixed micelles. However, this system does not simulate the membrane phospholipid bilayer. Joanna M. Kwiatek and George M. Carman at Rutgers University have developed a system that mimics the nuclear/ER membrane to assess the PA phosphatase activity. This recent research was published in the Journal of Lipid Research. As a model, the researchers used PA phosphatase of the yeast Saccharomyces cerevisiae. In this system, the substrate PA is incorporated into liposomes composed of the major ER membrane phospholipids.
The results revealed that the PA phosphatase activity depends on the phospholipid composition of liposomes, but the composition did not have a major effect on the enzyme and liposomes interaction. Moreover, the activity was dependent on both the molar and surface concentrations of PA in liposomes. These results suggest that the enzyme operates along the nuclear/ER membrane in the organism.
The authors propose a hopping and scooting model for the action of the PA phosphatase. The phosphorylated form of the enzyme is dephosphorylated by the Nem1-Spo7 complex at the nuclear/ER membrane. The dephosphorylated PA phosphatase hops onto the membrane surface. Then it scoots along the membrane, binds to its substrate PA and catalyzes the dephosphorylation of PA to produce diacylglycerol. The enzyme then scoots along the membrane and binds another molecule of PA to carry out another round of catalysis.
The system employed in this study has great potential to be applied to study other interfacial phospholipid synthetic enzymes.
A potential target against mycobacteria
Mycobacterium tuberculosis, the bacteria that causes tuberculosis, has a unique cell wall characterized by the presence of mycolic acids. These are lipids that play a key role in the pathogenesis of TB, the world’s top infectious killer, which causes 1.5 million deaths each year, according to the World Health Organization.
Nguyen-Hung Le and colleagues at the universities of Toulouse and Montpellier in France have learned that mycolic acid biosynthesis is regulated by kinase-mediated phosphorylation. In recent research published in the Journal of Lipid Research, they used lipidomic and phosphoproteomic analysis of a Mycobacterium strain with altered Ser/Thr phosphorylation. The authors discovered that a Ser/Thr protein kinase, PknB, plays a key role in maintaining cell wall integrity and regulates synthesis and transport of mycolic acids. Overexpression of PknB affected growth and enhanced susceptibility to antibiotics in mycobacteria .
The authors also identified the mycobacterial membrane protein large 3, or MmpL3, as the substrate of PknB, and this phosphorylation is the major regulator in mycolic acid trafficking and cell wall biosynthesis. Mycobacteria require MmpL3 to transport mycolic acids, and it is the proposed target of many anti-mycobacterial inhibitors under development.
These findings contribute to the understanding of mycolic acid biosynthesis, which is important to the development of new anti-mycobacterial compounds.
Ceramide reduction could be used to treat alcoholic liver disease
Alcoholic liver disease, a condition in humans caused by alcohol overconsumption, progresses from alcoholic steatosis (fatty liver) to fibrosis and cirrhosis. In alcoholic liver disease patients, the liver ceramides are increased. Ceramides are lipids that promote apoptosis and impair insulin signaling and cell growth. Researchers previously have shown that the reduction of ceramide prevents alcoholic steatosis and glucose intolerance in mice.
Jason Correnti from the Carr lab at the University of Pennsylvania and a team from several U.S. universities have demonstrated that ceramide reduction improves alcoholic liver disease in a mouse model. In a recent paper published in the Journal of Lipid Research, the researchers write that they overexpressed acid ceramidase, by an inducible system, to reduce hepatic ceramides in chronically alcohol-fed mice. They observed that ceramide reduction reverses alcohol-induced insulin resistance and improves alcoholic steatosis. Ceramidase induction decreases hepatic ceramides, and reduction of ceramide promotes very low-density lipoprotein secretion and increases lipophagy, which is the autophagic degradation of intracellular lipid droplets. Moreover, they observed decreased oxidative and lipotoxic stress in the mice.
The authors observed that a hepatic-specific acid-ceramidase is induced in human alcoholic liver disease. Therefore, the overexpression of acid-ceramidase is relevant in this disease. The results suggest a potential role for hepatic ceramide inhibition in preventing alcoholic liver disease.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

