Researchers investigate self-regulation of an enzyme with critical cellular functions
The lab of Kathy Gould at Vanderbilt University School of Medicine used a multidisciplinary approach that included structural biology, biochemistry and molecular biology to investigate the regulation of the CK1 enzyme family. The research was published in the journal Molecular Cell.
The work was led by postdoc Sierra Cullati and carried out in conjunction with research assistant professor Jun-Song Chen and scientists from Goethe University and the Structural Genomics Consortium in Frankfurt, Germany, and from Harvard University,
CK1 enzymes are a family of multifunctional kinases — enzymes that can phosphorylate, or add phosphate groups to, other proteins — that are critical for several cellular functions including DNA repair, endocytosis and mitotic checkpoint signaling. Regulation of CK1 enzymes is exceptionally important as dysfunction of these enzymes contributes to several conditions that include cancer, neurodegenerative diseases and sleep disorders.
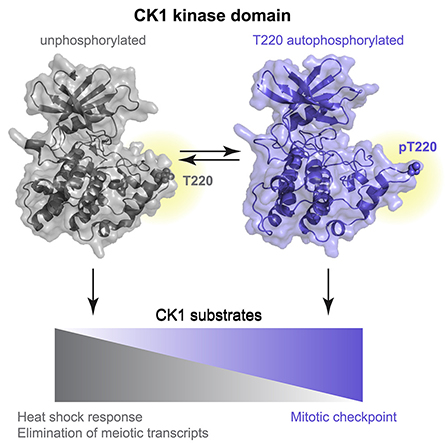
There are seven CK1 enzymes in mammals that perform different functions, but they are highly conserved in their catalytic domain, the region responsible for phosphorylation. Gould and colleagues found that one mechanism of CK1 activity, and thus one mechanism of regulation, is the self-phosphorylation of a conserved amino acid residue in its catalytic domain.
The researchers further investigated how this self-phosphorylation regulates activity and discovered that phosphorylation at this site altered the substrate specificity of CK1 enzymes. Substrate specificity refers to the determination of which other proteins the CK1 kinases will phosphorylate, which in turn determines which pathways within a cell get activated. In general, the phosphorylation state of CK1 enzymes controls their function — or dysfunction — within a cell. Determining which pathways are controlled by the phosphorylated versus non-phosphorylated states of the enzymes is a step toward the development of better treatments with fewer side effects for the diseases caused by enzyme dysfunction.
The Gould lab and collaborators hope to build upon this work by determining other sites of CK1 self-phosphorylation and investigating the pathways they regulate; there are several potential self-phosphorylation sites clustered together on one end of the protein, for example, that intrigue the researchers. Additionally, they plan to investigate how the discovered phosphorylation sites work together to provide additional control under different cellular conditions, such as cellular stress.
This article was republished with permission from the Vanderbilt School of Medicine. Read the original.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

