How lunar cycles guide the spawning of corals, worms and more
It’s evening at the northern tip of the Red Sea, in the Gulf of Aqaba, and Tom Shlesinger readies to take a dive. During the day, the seafloor is full of life and color; at night it looks much more alien. Shlesinger is waiting for a phenomenon that occurs once a year for a plethora of coral species, often several nights after the full moon.
Guided by a flashlight, he spots it: coral releasing a colorful bundle of eggs and sperm, tightly packed together. “You’re looking at it and it starts to flow to the surface,” Shlesinger says. “Then you raise your head, and you turn around, and you realize: All the colonies from the same species are doing it just now.”
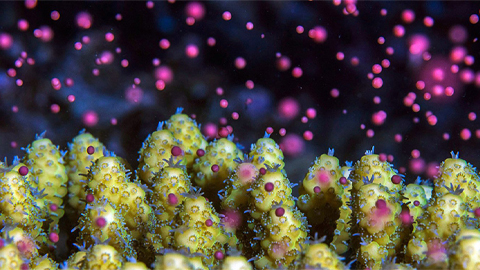
Some coral species release bundles of a pinkish- purplish color, others release ones that are yellow, green, white or various other hues. “It’s quite a nice, aesthetic sensation,” says Shlesinger, a marine ecologist at Tel Aviv University and the Interuniversity Institute for Marine Sciences in Eilat, Israel, who has witnessed the show during many years of diving. Corals usually spawn in the evening and night within a tight time window of 10 minutes to half an hour. “The timing is so precise, you can set your clock by the time it happens,” Shlesinger says.
Moon-controlled rhythms in marine critters have been observed for centuries. There is calculated guesswork, for example, that in 1492 Christopher Columbus encountered a kind of glowing marine worm engaged in a lunar-timed mating dance, like the “flame of a small candle alternately raised and lowered.” Diverse animals such as sea mussels, corals, polychaete worms and certain fishes are thought to synchronize their reproductive behavior by the moon. The crucial reason is that such animals — for example, over a hundred coral species at the Great Barrier Reef — release their eggs before fertilization takes place, and synchronization maximizes the probability of an encounter between eggs and sperm.
How does it work? That has long been a mystery, but researchers are getting closer to understanding. They have known for at least 15 years that corals, like many other species, contain light-sensitive proteins called cryptochromes, and have recently reported that in the stony coral, Dipsastraea speciosa, a period of darkness between sunset and moonrise appears key for triggering spawning some days later.
Now, with the help of the marine bristle worm Platynereis dumerilii, researchers have begun to tease out the molecular mechanism by which myriad sea species may pay attention to the cycle of the moon.
The bristle worm originally comes from the Bay of Naples but has been reared in laboratories since the 1950s. It is particularly well-suited for such studies, says Kristin Tessmar-Raible, a chronobiologist at the University of Vienna. During its reproductive season, it spawns for a few days after the full moon: The adult worms rise en masse to the water surface at a dark hour, engage in a nuptial dance and release their gametes. After reproduction, the worms burst and die.
The tools the creatures need for such precision timing — down to days of the month, and then down to hours of the day — are akin to what we’d need to arrange a meeting, says Tessmar-Raible. “We integrate different types of timing systems: a watch, a calendar,” she says. In the worm’s case, the requisite timing systems are a daily — or circadian — clock along with another, circalunar clock for its monthly reckoning.
To explore the worm’s timing, Tessmar-Raible’s group began experiments on genes in the worm that carry instructions for making cryptochromes. The group focused specifically on a cryptochrome in bristle worms called L-Cry. To figure out its involvement in synchronized spawning, they used genetic tricks to inactivate the l-cry gene and observe what happened to the worm’s lunar clock. They also carried out experiments to analyze the L-Cry protein.
Though the story is far from complete, the scientists have evidence that the protein plays a key role in something very important: distinguishing sunlight from moonlight. L-Cry is, in effect, “a natural light interpreter,” Tessmar-Raible and coauthors write in a 2023 overview of rhythms in marine creatures in the Annual Review of Marine Science.

The role is a crucial one, because in order to synchronize and spawn on the same night, the creatures need to be able to stay in step with the patterns of the moon on its roughly 29.5-day cycle — from full moon, when the moonlight is bright and lasts all night long, to the dimmer, shorter-duration illuminations as the moon waxes and wanes.
When L-Cry was absent, the scientists found, the worms didn’t discriminate appropriately. The animals synchronized tightly to artificial lunar cycles of light and dark inside the lab — ones in which the “sunlight” was dimmer than the real sun and the “moonlight” was brighter than the real moon. In other words, worms without L-Cry latched onto unrealistic light cycles. In contrast, the normal worms that still made L-Cry protein were more discerning and did a better job of synchronizing their lunar clocks correctly when the nighttime lighting more closely matched that of the bristle worm’s natural environment.
The researchers accrued other evidence, too, that L-Cry is an important player in lunar timekeeping, helping to discern sunlight from moonlight. They purified the L-Cry protein and found that it consists of two protein strands bound together, with each half holding a light-absorbing structure known as a flavin. The sensitivity of each flavin to light is very different. Because of this, the L-Cry can respond to both strong light akin to sunlight and dim light equivalent to moonlight — light over five orders of magnitude of intensity — but with very different consequences.
After four hours of dim “moonlight” exposure, for example, light-induced chemical reactions in the protein — photoreduction — occurred, reaching a maximum after six hours of continuous “moonlight” exposure. Six hours is significant, the scientists note, because the worm would only encounter six hours’ worth of moonlight at times when the moon was full. This therefore would allow the creature to synchronize with monthly lunar cycles and pick the right night on which to spawn. “I find it very exciting that we could describe a protein that can measure moon phases,” says Eva Wolf, a structural biologist at IMB Mainz and Johannes Gutenberg University Mainz, and a collaborator with Tessmar-Raible on the work.
How does the worm know that it’s sensing moonlight, though, and not sunlight? Under moonlight conditions, only one of the two flavins was photoreduced, the scientists found. In bright light, by contrast, both flavin molecules were photoreduced, and very quickly. Furthermore, these two types of L-Cry ended up in different parts of the worm’s cells: the fully photoreduced protein in the cytoplasm, where it was quickly destroyed, and the partly photoreduced L-Cry proteins in the nucleus.
All in all, the situation is akin to having “a highly sensitive ‘low light sensor’ for moonlight detection with a much less sensitive ‘high light sensor’ for sunlight detection,” the authors conclude in a report published in 2022.
Many puzzles remain, of course. For example, though presumably the two distinct fates of the L-Cry molecules transmit different biological signals inside the worm, researchers don’t yet know what they are. And though the L-Cry protein is key for discriminating sunlight from moonlight, other light-sensing molecules must be involved, the scientists say.
In a separate study, the researchers used cameras in the lab to record the burst of swimming activity (the worm’s “nuptial dance”) that occurs when a worm sets out to spawn, and followed it up with genetic experiments. And they confirmed that another molecule is key for the worm to spawn during the right one- to two-hour window — the dark portion of that night between sunset and moonrise — on the designated spawning nights.
Called r-Opsin, the molecule is extremely sensitive to light, the scientists found — about a hundred times more than the melanopsin found in the average human eye. It modifies the worm’s daily clock by acting as a moonrise sensor, the researchers propose (the moon rises successively later each night). The notion is that combining the signal from the r-Opsin sensor with the information from the L-Cry on what kind of light it is allows the worm to pick just the right time on the spawning night to rise to the surface and release its gametes.
Resident timekeepers
As biologists tease apart the timekeepers needed to synchronize activities in so many marine creatures, the questions bubble up. Where, exactly, do these timekeepers reside? In species in which biological clocks have been well studied — such as Drosophila and mice — that central timekeeper is housed in the brain. In the marine bristleworm, clocks exist in its forebrain and peripheral tissues of its trunk. But other creatures, such as corals and sea anemones, don’t even have brains. “Is there a population of neurons that acts as a central clock, or is it much more diffuse? We don’t really know,” says Ann Tarrant, a marine biologist at the Woods Hole Oceanographic Institution who is studying chronobiology of the sea anemone Nematostella vectensis.
Scientists are also interested in knowing what roles are played by microbes that might live with marine creatures. Corals like Acropora, for example, often have algae living symbiotically within their cells. “We know that algae like that also have circadian rhythms,” Tarrant says. “So when you have a coral and an alga together, it’s complicated to know how that works.”
Researchers are worried, too, about the fate of spectacular synchronized events like coral spawning in a light-polluted world. If coral clock mechanisms are similar to the bristle worm’s, how would creatures be able to properly detect the natural full moon? In 2021, researchers reported lab studies demonstrating that light pollution can desynchronize spawning in two coral species — Acropora millepora and Acropora digitifera — found in the Indo-Pacific Ocean.
Shlesinger and his colleague Yossi Loya have seen just this in natural populations, in several coral species in the Red Sea. Reporting in 2019, the scientists compared four years’ worth of spawning observations with data from the same site 30 years earlier. Three of the five species they studied showed spawning asynchrony, leading to fewer — or no — instances of new, small corals on the reef.
Along with artificial light, Shlesinger believes there could be other culprits involved, such as endocrine-disrupting chemical pollutants. He’s working to understand that — and to learn why some species remain unaffected.
Based on his underwater observations to date, Shlesinger believes that about 10 of the 50-odd species he has looked at may be asynchronizing in the Red Sea, the northern portion of which is considered a climate-change refuge for corals and has not experienced mass bleaching events. “I suspect,” he says, “that we will hear of more issues like that in other places in the world, and in more species.”
This article originally appeared in Knowable Magazine, an independent journalistic endeavor from Annual Reviews.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

