Team discovers how to sabotage antibiotic-resistant ‘superbugs’
Antibiotic-resistant “superbugs” that can defeat efforts to kill them are an urgent public health crisis, and according to the CDC, more than 2.8 million antibiotic-resistant infections occur each year. Researchers across the world are scrambling to meet the challenge. Recently, a collaborative team of researchers lead by the University of Massachusetts Amherst and including scientists from the biopharmaceutical company Microbiotix, announced in the journal ACS Infectious Diseases that they had successfully learned how to sabotage a key piece of machinery, called the Type 3 secretion system, that pathogens use to infect their host cells. Furthermore, the team reports that they have developed a test to identify the next-generation drugs to target this vulnerable cellular machinery and make real gains in public health.
The typical strategy when treating microbial infections is to blast the pathogen with an antibiotic drug, which works by getting inside the harmful cell and killing it. This is not as easy as it sounds, because any new antibiotic needs to be both water soluble, so that it can travel easily through the blood stream, and oily, in order to cross the pathogenic cell’s first line of defense, the cellular membrane. Water and oil, of course, don’t mix, and it’s difficult to design a drug that has enough of both characteristics to be effective.
The difficulty doesn’t stop there, either, because pathogenic cells have developed something called an “efflux pump,” that can recognize antibiotics and then safely excrete them from the cell, where they can’t do any harm. If the antibiotic can’t overcome the efflux pump and kills the cell, then the pathogen “remembers” what that specific antibiotic looks like and develops additional efflux pumps to efficiently handle it—in effect, becoming resistant to that particular antibiotic.
One path forward is to find a new antibiotic, or combinations of them, and try to stay one step ahead of the superbugs.
“Or, we can shift our strategy,” says Alejandro Heuck, associate professor of biochemistry and molecular biology at UMass Amherst and the paper’s senior author. “I am a chemist, and I’ve always been very interested in understanding how chemical molecules interact with living organisms. In particular, I have been focusing my research on the molecules that make communication possible between a pathogen and the host cell it wants to invade.”
Heuck and his colleagues have been particularly interested in a communication system called the Type 3 secretion system, which, so far, appears to be an evolutionary adaptation unique to pathogenic microbes.
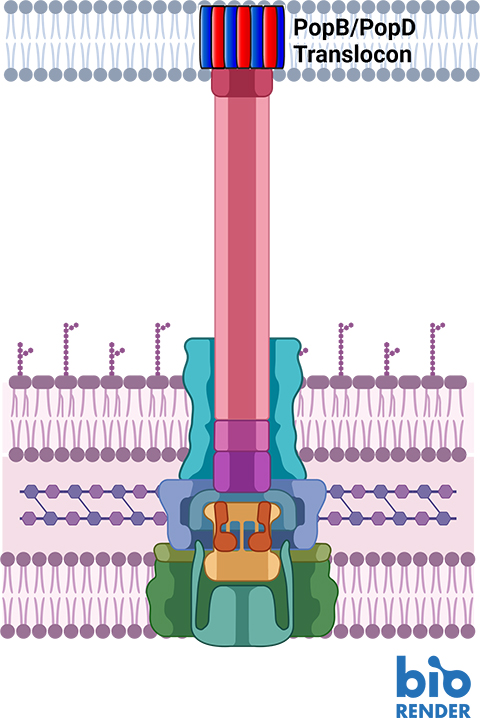
Like the pathogenic cell, host cells also have thick, difficult-to-penetrate cell walls. In order to breach them, pathogens have developed a syringe-like machine that first secretes two proteins, known as PopD and PopB. Neither PopD nor PopB individually can breach the cell wall, but the two proteins together can create a “translocon” — the cellular equivalent of a tunnel through the cell membrane. Once the tunnel is established, the pathogenic cell can inject other proteins that do the work of infecting the host.

This entire process is called the Type 3 secretion system—and none of it works without both PopB and PopD. “If we don’t try to kill the pathogen,” says Heuck, “then there’s no chance for it to develop resistance. We’re just sabotaging its machine. The pathogen is still alive; it’s just ineffective, and the host has time to use its natural defenses to get rid of the pathogen.”
The question, then, is how to find the molecule that can block the assembly of the translocon?
Sometimes, solutions come to scientists in those “lightbulb moments” when suddenly everything makes sense. In this case, it was more of a lightning bug moment.
Heuck and his colleagues realized that an enzyme class called the luciferases—similar to the ones that cause lightning bugs to glow at night — could be used as a tracer. They split the enzyme into two halves. One half went into the PopD/PopB proteins, and the other half was engineered into a host cell.
These engineered proteins and hosts can be flooded with different chemical compounds. If the host cell suddenly lights up, that means that PopD/PopB successfully breached the cellular wall, reuniting the two halves of the luciferase, causing them to glow. But if the cells stay dark? “Then we know which molecules break the translocon,” says Heuck.
Heuck is quick to point out that his team’s research has not only obvious applications in the world of pharmaceuticals and public health, but that it also advances our understanding of exactly how microbes infect healthy cells. “We wanted to study how pathogens worked,” he says, “and then suddenly we discovered that our findings can help solve a public-health problem.”
This article was originally published on the University of Massachusetts website. Read the original here.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

