A new hotspot for cyclooxygenase inhibition
Prostaglandins, or PGs, are critical players in such physiological housekeeping functions as the regulation of renal water and sodium metabolism, blood clotting, parturition, and stomach acid secretion. These potent lipid signaling molecules are derived from the oxygenation of arachidonic acid by prostaglandin endoperoxide H synthases 1 and 2, commonly called cyclooxygenase-1 and -2, or COX-1 and COX-2.
Abnormal changes in PG production contribute to disease pathologies including inflammation and cancer, so researchers view COX-1 and COX-2 as pharmacological targets. Indeed, nonselective nonsteroidal anti-inflammatory drugs, or NSAIDs, such as ibuprofen, inhibit both isoforms, while coxibs such as Celebrex selectively inhibit COX-2.
COX enzymes are sequence homodimers; each subunit consists of physically distinct cyclooxygenase and peroxidase active sites linked by a bridging heme moiety. In solution, the enzymes function as heterodimers, such that only one subunit is catalytically active at a time.
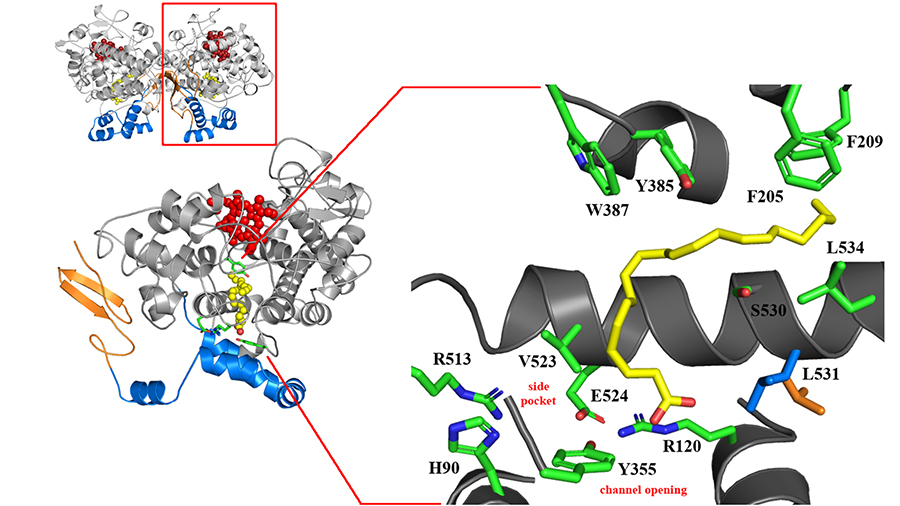
Structurally, COX-1 and COX-2 are virtually identical. However, subtle differences drive isoform-specific substrate specificity and inhibition. Among these are the substitutions of cyclooxygenase channel residues Ile-434, His-513 and Ile-523 in COX-1 for Val-434, Arg-513 and Val-523 in COX-2, which result in the formation of a unique side pocket and increased volume of the cyclooxygenase active site in COX-2. This side pocket, with Arg-513 at its base, has been exploited in the design of coxibs aimed at reducing the gastrointestinal side effects caused by conventional NSAIDs.
Many researchers focus on time-dependent inhibition of COX-2, working to identify the conformational changes that drive the transitions between the kinetically observable inhibitor binding states. These studies originally focused on the COX-2 side pocket, but recent molecular dynamics simulations identified a different region of the active site critical for aspirin and Celebrex to inhibit COX-2. These simulations identified rotation of the side chain of the residue Leu-531, opposite the side pocket, as a way to expand further the volume of the cyclooxygenase channel. Indeed, crystal structures of COX-2 have provided evidence for the existence of alternate rotamer conformations for this side chain when different ligands are bound.
To evaluate the importance of the conformational flexibility of the side chain of Leu-531, our research group generated L531F and L531N mutations and showed that these substitutions reduced aspirin and Celebrex’s inhibition of COX-2. Thus, expansion of the active site volume via the flexibility associated with the side chain of Leu-531 allows Celebrex to achieve a high-affinity binding state and facilitates formation of the initial noncovalent complex in the case of aspirin acetylation.
Deciphering the protein conformational motions associated with COX catalysis and inhibition is the next major frontier in studying this enzyme. If we understand how the binding of substrates, allosteric activators and inhibitors induce conformational motions to modulate COX activity, we will be closer to developing and repurposing drugs for maximum efficacy with minimal risks.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

