World Osteoporosis Day
The first two years of medical school, during which medical students are inundated with floods of information on all the various ways in which someone’s health can go awry, are enough to turn even the most stoic logician into a veritable hypochondriac.
For me, and I daresay for many of my 20-some year-old classmates, perhaps the most worrisome information came close to the end of our classroom-based curriculum. After 10 or so months drilling into our heads all possible maladies of the heart, circulatory system, respiratory system, digestive tract, urinary tract, reproductive tract, skin, and brain, we had just launched into our rheumatology unit when our professors informed us that we had only until age 30 to build up our peak bone density. After this, we would spend the rest of our lives gradually losing bone density — perhaps descending into osteoporosis, and all of its debilitating sequelae, if we didn’t start our descent from a high enough “altitude.”
And the way to climb higher on the bone density mountain? Physical activity.
My classmates shifted uneasily in their seats. Although there were a few devoted exercise enthusiasts among us, everyone else was suddenly painfully aware of the hours and hours they spent glued to a seat: listening to lectures, studying their notes, doing practice questions for the first installment of our board exams, or — for the somewhat less studious — playing video games.
Our demanding schedule provided many convenient excuses to avoid hitting the gym. Although as aspiring physicians we all knew the value of regular exercise to overall good health, its connection to the prevention of other illnesses — cancer, heart disease, depression — was vague and distant enough to let the excuses prevail. But now we were presented with an all-too-direct threat to our health and an all-too-actionable preventative measure, one that couldn’t be put off for later as we all inched (or rather hurtled) ever closer to the “30” cutoff.
Although I can’t attest to the actions of my classmates, I, for one, was duly guilted into recruiting a friend — one of the aforementioned exercise enthusiasts — to help me create a five-day-a-week exercise schedule, complete with cardio and strength training. In an admirable showing for someone as exercise-averse as me, I managed to stick to this schedule for the entire two weeks that remained in the semester before the uneasy fear instilled in me by our rheumatology professors was eclipsed by the more immediate fear of failing my board exam, allowing me to swap my exercise schedule for a much more intensive study schedule.
More than five years later (yes, I am still in school—M.D.–Ph.D. students have a long road to travel), I have so far been unable to recapture that fervent motivation even though “30” is now just around the corner. But occasionally a creeping anxiety still passes through my still-salvageable bones when I remember that the bone density clock is ticking, because osteoporosis — a disease significant enough in prevalence and individual impact to merit its own awareness day each year on Oct. 20 — is not to be taken lightly.
Here, I’ll take a closer look at what osteoporosis entails before digging into some recent research articles on the subject. With any luck, it will be enough to push me — and maybe some of you — into an exercise routine that will help us reach the peak of the bone density mountain before we begin our inevitable descent. And for those readers past 30, you’re not completely off the hook: Exercise can still help slow the descent.
What is osteoporosis, and what symptoms does it cause?
Although we often think of bone as a static support system for the rest of our organs and tissues, it is actually composed of dynamic, living tissue. The majority of bone volume is occupied by a strong matrix of organic collagen fibers (along with a few other proteins) interspersed with inorganic calcium and phosphate crystals (mostly in a form called hydroxyapatite), which harden the matrix. Nestled within this matrix are cells — mainly osteoblasts and osteoclasts — that continuously remodel bone tissue by respectively laying down new and breaking down existing matrix.
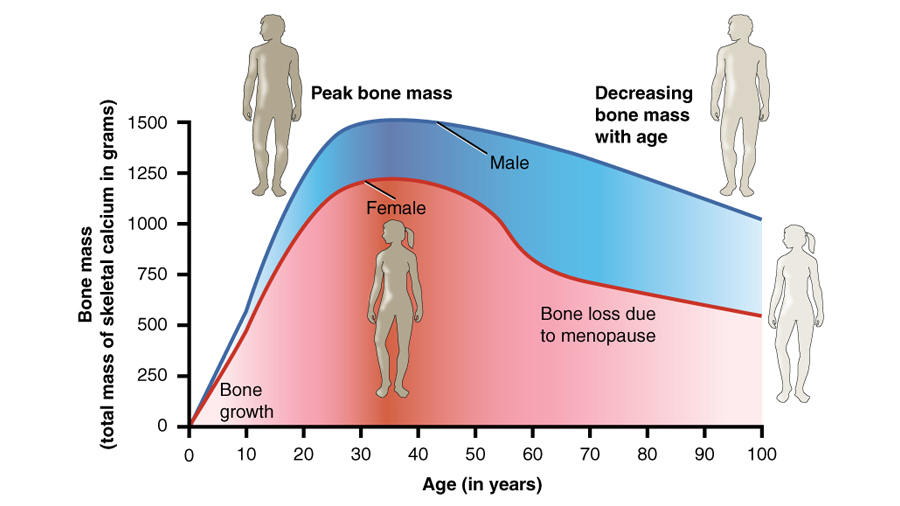
The balance of these two activities — bone formation and resorption — determines bone growth and density and shifts naturally over a person’s lifespan (as shown the figure). Children and teenagers enjoy a brisk pace of osteoblast activity that eclipses osteoclast activity enough to cause lengthening at still-open growth plates on long bones in addition to densifying of bone. Young adults up until about age 30 continue to benefit from a balance tipped in favor of bone formation, resulting in continued densifying of bone. After this period, bone resorption accelerates, outstripping formation and leading to gradual loss of bone density and therefore weakening of bones.
If bone density drops too low, the weakened bones become susceptible to fractures from even mild impacts. Such “fragility fractures” are the main consequence of osteoporosis. Further sequelae can result from these fractures and impact quality of life: fractures in long bones and hips can impair motility (with the latter often requiring risky, sometimes fatal surgery), wrist fractures can hinder use of the hands, rib fractures can cause pain with each breath as they heal, and vertebral fractures can elicit chronic pain and bend the back into a permanently stooped posture.
A collection of stories from osteoporosis patients of many walks of life and from many different cultural backgrounds provides a more intimate look at the deleterious impacts osteoporosis can have on everyday life.
Osteoporosis is often not diagnosed until a usually elderly individual suffers a tell-tale fracture, often from a fall. However, bone mineral density (BMD) can be measured in the clinic, a screening measure currently recommended for elderly adults and other at-risk populations, by means of dual energy X-ray absorptiometry (DEXA). DEXA measures the absorption of two X-ray beams of different energy levels by the bones, usually those of the lumbar spine and hip but sometimes substituting the forearm if one or both are inaccessible, in order to calculate BMD. For post-menopausal women and men over age 50, results are compared to standard data for healthy 30-year-olds, with or without sex- and ethnicity-matching (for younger men and pre-menopausal women, standard data for healthy age-, sex-, and ethnicity-matched individuals is used). If the BMD is between 1.0 and 2.5 standard deviations below average, a person is diagnosed with osteopenia (also called simply “low bone mass” or “low bone density”), and if the BMD is 2.5 or more standard deviations below average, the verdict is osteoporosis.
How common is osteoporosis?
The short answer is very common — one in three women and one in five men over 50 will experience a hallmark “fragility fracture.” Osteoporotic fractures will only become more common as the world’s elderly population balloons. The 2016 statistic of 8.5% of the global population over 65 years of age could swell to 17% by 2050, an estimated 1.6 billion individuals. The prevalence of osteoporosis exerts enormous socio-economic pressures, particularly on caregivers and healthcare systems.
What are risk factors for osteoporosis?
Several factors can put a person at risk for osteoporosis. Some of these are immutable facts of your biology — they cannot be helped — but others are under your control, meaning you can make lifestyle changes to temper your risk. Let’s take a look at established risk factors in each of these categories:
Immutable risk factors (determine your baseline risk):
-
Sex: Women are at a higher risk than men for developing osteoporosis. Not only do women typically achieve lower peak bone mass, but they also experience accelerated bone loss in the first few years after menopause due to hormonal changes that affect the speed of bone resorption.
-
Age: Increasing age correlates with increasing risk for osteoporosis due to cumulative bone loss over time.
-
Body size: Smaller bodies typically have thinner bones, especially in women, elevating the risk of osteoporosis.
-
Genetics: Women of European and Asian ancestry are at higher risk of osteoporosis than women of other ethnicities, and some additional genetic factors can also increase fracture risk
Mutable risk factors (may give you room for risk reduction):
-
Low body weight: Whether due to malnutrition, chronic illness, or an eating disorder, a low body-mass index dampens bone density, especially when accompanied by amenorrhea in women. Increasing body weight to a normal BMI mitigates the risk.
-
Calcium and vitamin D intake: Deficiencies of either can hasten bone loss. Taking supplements, getting some sun (for vitamin D, at least), and consuming calcium-rich foods can help.
-
Sedentary lifestyle: Just like muscles, it’s “use it or lose it” for bones, too. They become stronger with regular exercise, particularly of the weight-bearing and resistance varieties (think walking, hiking, jogging, stair climbing, and lifting weights), as these require using your bones to push against gravity. Exercise also helps with muscle tone and coordination, which can prevent the falls that often lead to “fragility fractures” in people who already have weakened bones.
-
Smoking: Adding to an already formidable list of horrible effects smoking has on the body, there is an established link between smoking and low bone density. Mechanistically, this may be related to production of proteins that boost osteoclast production. Practically, quitting is the best way to reduce risk (and not only of osteoporosis but myriad other ailments as well).
-
Excessive alcohol consumption: Perhaps surprisingly, too much alcohol can wreak havoc on your bones by altering calcium balance and messing with hormone production. You’re also more likely to fall when you’re under the influence, increasing the opportunity for fractures to occur if your bones are already weak.
-
Certain medications: If taken for long periods of time, some medications, such as glucocorticoids, anticonvulsants and certain cancer treatments, can accelerate bone loss. If osteoporosis is a concern, swapping these out for alternative treatments when available may help reduce risk.
Any of these risk factors sound uncomfortably familiar? Think you — or someone close to you — may have some of these risk factors? You can check your risk for developing osteoporosis and see some helpful tips for reducing your risk using this handy online questionnaire.
Can osteoporosis be prevented or treated?
For prevention, look no farther than the list of mutable risk factors above. Minimizing these is the best way to keep your bone density in a healthy range. For patients who already have severely diminished bone density, there are a few treatment options and practical measures to consider that can help reduce fracture risk, stem bone loss, and even increase bone density to a certain extent.
Maintaining a healthy lifestyle is an important cornerstone of treatment — a balanced diet with sufficient calcium and vitamin D coupled with regular (gentle) exercise supports bone strength. Fall prevention is another key consideration, as many fractures — especially dangerous hip fractures — result from falls. Simple but effective measures include using a cane or walker, avoiding slippery surfaces, wearing supportive and traction-providing footwear, and making sure staircases are equipped with handrails on both sides.
When lifestyle changes and fall prevention efforts aren’t quite enough, patients can choose from several therapeutics based on their specific needs: antiresorptive medications such as bisphosphonates, estrogen agonists/antagonists (EAAs; also called selective estrogen receptor modulators or SERMs), estrogens, calcitonin, and the biologic denosumab (an inhibitor of RANKL, an osteoblast protein that promotes osteoclast activity) slow the rate of bone resorption, while anabolic medications, which currently include a parathyroid hormone (PTH) analog and a PTH-related protein analog, stimulate osteoblasts to lay down new bone.
With this list of current treatment options as a nice segue, let’s move on to exploring some recent research findings related to osteoporosis. Many focus on elucidating the basic biology of the bone cells whose balance is disrupted in osteoporosis and the signaling pathways that govern their respective functions, potentially laying the groundwork for the development of new therapeutics.
STAT3 is critical in bone catabolism
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor involved in many key cellular processes, including maintenance of bone homeostasis. Previous in vitro studies point to a role for STAT3 in stimulating generation of osteoclasts. To investigate the effects of STAT3 on osteoclasts in vivo, Yang et al. created a mouse model in which the gene for STAT3 could be conditionally deleted in osteoclasts specifically. Upon osteoclastic stat3 deficiency, bone mass was increased, indicating decreased osteoclast generation and/or activity. The authors further found that in bone marrow macrophages (BMMs) — the progenitors of osteoclasts—stat3 deficiency led to reduced differentiation into osteoclasts, which they linked to decreased levels of nuclear factor of activated T cells (NFATc1). They showed that STAT3 binds to the promoter of NFATc1 to drive its transcription, explaining the reduced NFATc1 levels observed in the absence of STAT3. Their results — published in the Journal of Biological Chemistry — demonstrate the key role STAT3 plays in osteoclast differentiation in vivo and suggest that components of the pathway by which it mediates this, such as NFATc1, could serve as targets in the treatment of osteoporosis.
Cytokine blocks differentiation into osteoblasts
Osteoblasts, the cells responsible for bone formation, are derived from multipotent mesenchymal stem cells (MSCs), which express a protein called leukemia inhibitory factor receptor β (LIFRβ) on their surface. When the gene encoding for this protein is mutated, the result is Stüve–Wiederman syndrome, characterized by marked skeletal deformities and osteoporosis; this indicates that signaling through LIFRβ is critical for proper bone formation and maintenance of bone density. LLIFRβ serves as a receptor for cardiotrophin-like cytokine (CLCK1), but mutations or deficiencies in CLCK1 do not appear to cause bone manifestations, meaning it is likely not among the LIFRβ ligands that promote bone formation and maintenance. Nahlé et al. thus hypothesized that CLCK1 may have a different function with respect to osteoblast differentiation and set out to investigate its effects on MSCs. They ultimately found that CLCK1 binds to mouse MSCs in vitro and triggers a signaling pathway that inhibits the generation of osteoblasts. Their results, published in JBC, point to inhibition of CLCK1, for example using blocking antibodies, as a potential therapeutic strategy for osteoporosis.
IL-27 mitigates bone loss in mice
Recent studies have pointed to the involvement of inflammatory processes in the pathogenesis of osteoporosis. In this study published in JBC, Shukla et al. explored the role of the anti-inflammatory cytokine interleukin 27 (IL-27) in osteoporosis by using it to treat ovariectomized, estrogen-deficient mice, a model for post-menopausal osteoporosis. The treatment preserved bone structure and also inhibited osteoblast apoptosis and suppressed osteoclastogenesis. In effort to extend some of their findings to human patients, the researchers tested serum levels of IL-27 in healthy, osteopenic, and osteoporotic post-menopausal women and found a slight positive correlation between IL-27 levels and bone mineral density. Overall, these results highlight a potential application for IL-27 in treating post-menopausal osteoporosis.
A focus on anabolic osteoporosis drugs
There are currently two anabolic — or bone-building — medications approved by the U.S. Food and Drug Administration for osteoporosis: Teriparatide is a parathyroid hormone (PTH) analog, and abaloparatide is a parathyroid hormone-related protein (PTHrP) analog. PTH is normally secreted in response to low serum calcium levels and stimulates bone resorption to release calcium from bone tissue, as does PTHrP; chronic exposure to either leads to bone resorption. Use of their analogs to treat osteoporosis would therefore seem counterintuitive, but intermittent administration actually has the opposite effect, preferentially stimulating bone formation. PTH, PTHrP, and their analogs bind to the PTH/PTHrP receptor type 1 (PTHR1) expressed on the surface of osteoblasts, setting off a complex signaling cascade, one effect of which is to elicit expression of RANKL, a surface protein that binds to another protein called RANK on the surface of osteoclasts to stimulate bone reabsorption. Even though they all bind to PTHR1, previous studies have hinted that the effects of teriparatide, PTHrP and abaloparatide on the osteoblast may differ. Ricarte et al. investigated this question in a study published in JBC, focusing on differences in expression of the Rankl gene. The researchers treated osteoblasts with each of the three peptides in vitro and found that both PTHrP and abaloparatide induced lower levels of Rankl expression than teriparatide. They further elucidated differences in the signaling cascades triggered by each peptide that mediated differential Rankl expression. Induction of Rankl to a lesser degree by abaloparatide may explain why this drug is associated with lower levels of serum biomarkers for bone resorption in osteoporosis patients.
Osteoclasts in Hajdu Cheney syndrome
Hajdu Cheney syndrome (HCS) is an extremely rare genetic disorder caused by mutations in a receptor called NOTCH2 that prevent its proper breakdown, leading to continuous function. This results in numerous skeletal abnormalities, including osteoporosis. Histological examination of tissue from affected patients has pointed to evidence of an inflammatory response, prompting Yu and Canalis to hypothesize that tumor necrosis factor α (TNFα), a pro-inflammatory cytokine known to promote osteoclast generation and activity, might be involved in the pathogenesis of osteoporosis in HCS. To investigate whether the NOTCH2 mutations found in HCS enhance bone sensitivity to TNFα-mediated resorption, they treated a mouse model of HCS with TNFα and noted increased osteoclast number and activity compared to littermate controls. In vitro, TNFα also led to greater generation of osteoclasts from bone marrow-derived macrophages (BMMs) of HCS mice than from BMMs of control mice; this effect was prevented by blocking NOTCH2 activation. These results, published in JBC, demonstrate that the NOTCH2 mutations in HCS enhance the osteoclast-generating and -stimulating effects of TNFα, providing a possible explanation for the pathogenesis of osteoporosis in the syndrome.
Vitamin K and osteoporosis risk
Forms of vitamin K serve as co-factors for gamma-glutamyl carboxylase (GGCX), an enzyme that carboxylates poly-glutamate residues in numerous other important proteins. This GGCX-mediated carboxylation of glutamate residues increases calcium ion affinity, and subsequent binding of calcium elicits a shift to a more active conformation. Some such carboxylated proteins are involved in bone mineralization, and low physiologic levels of vitamin K have been associated with increased risk for osteoporosis. In a Journal of Lipid Research article, McDonald et al. reported development of an LC-MS/MS assay to quantify urinary metabolites of vitamin K and validated its sensitivity and precision. They also identified a novel urinary vitamin K metabolite. Given the link between low vitamin K levels and osteoporosis risk, vitamin K metabolites such as the ones measured in this study could potentially be used as urine biomarkers to identify patients at risk for osteoporosis.
Possible unintended consequences
In a more peripherally related study published in Molecular & Cellular Proteomics, Wierer et al. characterized the proteome of developing atherosclerotic plaques in the aortas of mice using mass spectrometry-based proteomics. They identified several novel proteins involved in atherosclerosis, including three subunits of the osteoclast V-ATPase complex, which were enriched in macrophages (cells that share a common lineage with osteoclasts). The osteoclast V-ATPase is employed by bone-resorbing osteoclasts to dissolve minerals, and the authors hypothesized that it may fight against plaque calcification in atherosclerosis. Linking their findings to osteoporosis, the authors noted that inhibition of V-ATPase has been considered as a strategy for treatment of osteoporosis; since many osteoporosis patients also suffer from atherosclerosis, use of such inhibitors could have an unintended detrimental effect on vascular health, as it could bolster plaque calcification.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.