Why is the 100-year-old BCG vaccine so broadly protective in newborns?
The century-old Bacille Calmette-Guérin (BCG) vaccine against tuberculosis is one of the world’s oldest and most widely used vaccines, used to immunize 100 million newborns every year. Given in countries with endemic TB, it has surprisingly been found to protect newborns and young infants against multiple bacterial and viral infections unrelated to TB. There’s even some evidence that it can reduce severity of COVID-19.
doi.org/10.1016/j.celrep.2022.110772
What’s special about BCG vaccine? How does it protect infants so broadly? It turns out little is known. To understand its mechanism of action, researchers at the Precision Vaccines Program at Boston Children’s Hospital partnered with the Expanded Program on Immunization Consortium (EPIC), an international team studying early life immunization, to collect and comprehensively profile blood samples from newborns immunized with BCG, using a powerful “big data” approach.
Their study, published online May 3 in Cell Reports, found that the BCG vaccine induces specific changes in metabolites and lipids that correlate with innate immune system responses. The findings provide clues toward making other vaccines more effective in vulnerable populations with distinct immune systems, such as newborns.
Small babies, big data
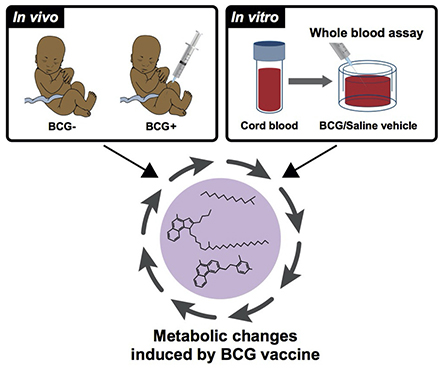
First author Joann Diray Arce, PhD, and her colleagues began with blood samples from low-birthweight newborns in Guinea Bissau who were enrolled in a randomized clinical trial to receive BCG either at birth or after a delay of six weeks. Both groups had small blood samples taken at four weeks (after BCG was given to the first group, and before it was given to the second group).
Using metabolomics and lipidomics, the team comprehensively profiled the impact of BCG immunization on the newborns’ blood plasma. They found that BCG vaccines given at birth changed metabolite and lipid profiles in newborns’ blood plasma in a pattern distinct from those in the delayed-vaccine group. The changes correlated with changes in cytokine production, a key feature of innate immunity.
The researchers had parallel findings when they tested BCG in cord blood samples from a cohort of Boston newborns and samples from a separate NIH/NIAID-funded Human Immunology Project Consortium study of newborns in The Gambia and Papua New Guinea.
“We now have some lipid and metabolic biomarkers of vaccine protection that we can test and manipulate in mouse models,” says Arce. “We studied three different BCG formulations and showed that they converge on similar pathways of interest. Reshaping of the metabolome by BCG may contribute to the molecular mechanisms of a newborn’s immune response.”
“A growing number of studies show that BCG vaccine protects against unrelated infections,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program and the study’s senior investigator. “It’s critical that we learn from BCG to better understand how to protect newborns. BCG is an ‘old school’ vaccine — it’s made from a live, weakened germ — but live vaccines like BCG seem to activate the immune system in a very different way in early life, providing broad protection against a range of bacterial and viral infections. There’s much work ahead to better understand that and use that information to build better vaccines for infants.”
The study was supported by the NIAID (U19AI118608, U01 AI124284), the Precision Vaccines Program at Boston Children’s Hospital, and the Mueller Health Foundation. Levy is a named inventor on several Boston Children's Hospital patents relating to human microphysiologic assay systems and vaccine adjuvants. Coauthors Scott McCulloch and Greg Michelotti are employees of Metabolon Inc. The other authors declare no competing financial interests.
This article was reprinted with permission from Boston Children's Hospital. Read the original.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

