Building bridges between basic and clinical research
Karin Bornfeldt always knew she wanted to do research. She has fond childhood memories of examining fossils, stuffed and mounted birds, and formalin-preserved snakes and fish in her father’s high school biology classroom in Sweden. Thanks to her parents’ encouragement, Bornfeldt said, “I grew up having a very strong connection to the natural world and being very curious about how things work.” She was drawn to medical research — although she might happily have studied ethology, plant physiology or other fields of biology.

As it happens, Bornfeldt became interested as a graduate student in how diabetes predisposes patients to heart disease. Atherosclerosis, a common complication of diabetes and the cause of heart attacks and strokes, arises when white blood cells squeeze in between an artery and its smooth-muscle sheath. Over time, these cells accumulate lipids and contribute to the development of cholesterol-filled lesions that can rupture or fissure and block blood flow. However, it is not well understood why Type 1 diabetes, which arises when the immune system attacks the pancreatic beta cells, and type 2 diabetes, which develops after insulin-secreting pancreatic b-cells are overstressed, can accelerate atherosclerosis.
Bornfeldt’s lab demonstrated that in diabetic mice, lipids are more important than glucose in accelerating atherosclerosis. She continues to probe the links between the two diseases as the deputy director of, and director of the diabetes complications research program at, the University of Washington’s Diabetes Institute. Since 2019, she also has served as an associate editor of the Journal of Lipid Research.
Bornfeldt recently discussed her work with ASBMB Today. This interview has been edited.
You study how diabetes accelerates atherosclerosis. How did you get interested in this area of research?
I have worked in this area since I was a graduate student back in Sweden. I studied with a diabetologist there, a physician–scientist named Hans Arnqvist. I was his only graduate student at the time, and I got to know his clinical team and some of his patients. When we met, his pager would often go off for patients who had had heart attacks or strokes. Sometimes they were very young. Before my graduate studies, I had not heard of women having heart attacks in their 30s, and it made me realize that the connection between diabetes and early heart disease was something that we urgently needed to understand and find treatments for.
At that time there weren’t any good animal models to study diabetes-accelerated atherosclerosis, the process that leads to cardiovascular disease. I thought better animal models would be needed in order to understand mechanisms and move the research area forward. As a graduate student, I learned a lot about diabetes, and I decided to pursue studies in atherosclerosis by moving to the University of Washington to train with Russell Ross, who was a leader in atherosclerosis research. I was also fortunate to study signal transduction in vascular cells with Ed Krebs.
After my postdoc period, I set up my own lab here at the University of Washington, and that’s when I had the opportunity to really focus on cardiovascular complications of diabetes by generating a mouse model of that disease. So I moved from studying vascular tissue and cultured cells to animals; we generated a new mouse model that we still use a lot to study mechanisms whereby diabetes promotes atherosclerosis. Those studies brought us to the understanding that diabetes affects several different stages of atherosclerotic lesions and that the mechanisms might be different for different stages of lesion progression.
More recently, my lab has taken another step toward human translational studies; we’re combining data that we get from large human cardiovascular outcome studies with the mechanistic mouse models that we have in the lab. We’re now basing our research on data from human studies to be sure that we’re studying the most important drivers of cardiovascular disease risk in diabetes in humans. It’s been a rewarding progression in my career, going from studying single cells in culture to animal models to translational and clinical studies.
How do you apply an insight from a large clinical trial to a mouse model?
A good example is work we did recently on apolipoprotein C3 in collaboration with Janet Snell–Bergeon at the University of Colorado. We obtained baseline samples from humans with Type 1 diabetes involved in a study called CACTI. These subjects didn’t have any cardiovascular disease when the plasma samples were collected, but they were followed over time so that we knew who developed a myocardial infarction later on. We used targeted mass spectrometry to identify proteins in those plasma samples that predicted who was going to have a cardiovascular event later in life. We identified apolipoprotein C3 as a risk factor, then silenced this protein in our diabetic mouse model to investigate if APOC3 is a causative factor or just a biomarker of increased atherosclerosis. It turned out to be a very strong causative factor: Animals without APOC3 were completely protected from atherosclerosis even when they were diabetic and had very high blood glucose levels. We think that APOC3 works by slowing the clearance of atherogenic remnant lipoprotein particles. We’re now starting similar studies in people with Type 2 diabetes and also looking for other proteins that are involved in the pathway of remnant clearance.
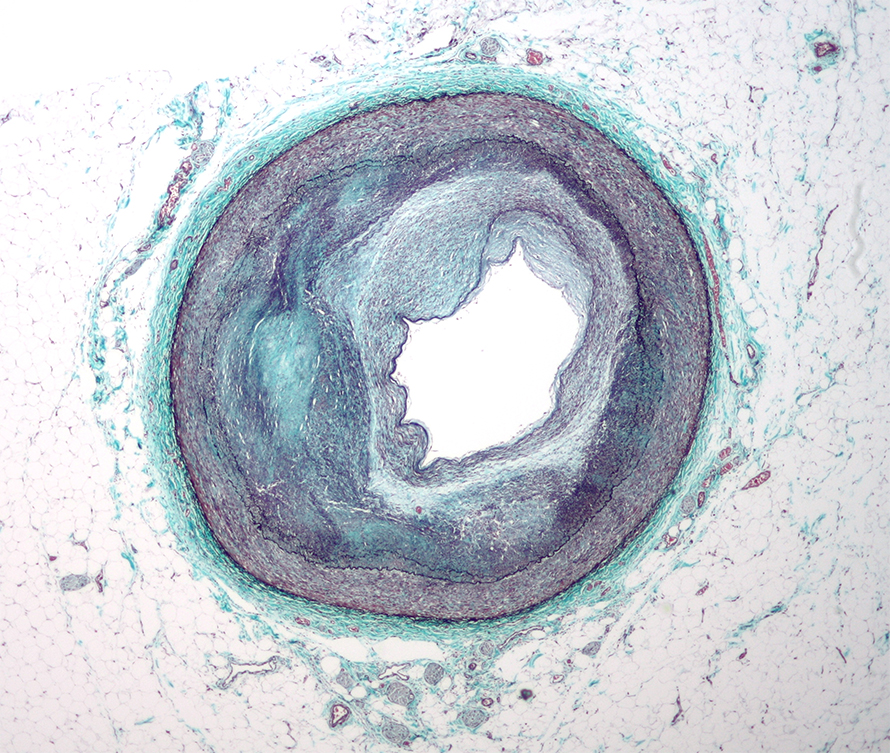
You mentioned the mouse model that you developed. Do I understand right that it’s a mouse with pancreatic b-cells that are inducibly attacked by its immune system?
Yes, that’s our main model of Type 1 diabetes. It’s a really reliable model that accelerates atherosclerosis. We have also used other, more traditional models in the lab, like fat feeding to induce insulin resistance and a b-cell toxin called streptozotocin, but the T-cell mediated b-cell destruction model is our go-to. As with humans with Type 1 diabetes, the diabetic mice need constant care — insulin injections, glucose checks and so on.
The main research area we’re focusing on right now is centered on a group of lipoprotein particles called remnant lipoprotein particles. We think that those remnants can penetrate the artery wall and accelerate atherosclerosis and that they play a particularly important role in diabetes. And it looks like proteins that increase the accumulation of these remnants in the artery wall are very important in cardiovascular disease progression. How those remnants are related to inflammation and changes in immune cells is another area that we’re very actively studying right now too.
Why are they called remnants?
They are remnants of triglyceride-rich lipoproteins. There are two types of triglyceride-rich lipoproteins. One type is very large particles called chylomicrons that come from the gut following a meal. The other type is very low-density lipoproteins that come from the liver between meals. Both are larger particles than LDL, but they are acted upon by lipases that make them smaller and smaller by hydrolyzing the triglycerides carried in these particles — and then they are called remnants. These remnants can be cleared by the liver, or they can accumulate in the artery wall and promote atherosclerosis.
Actually, there aren’t clear definitions of remnants or ways to measure them yet. As these particles become progressively smaller as they are hydrolyzed by lipases, they change in composition as well as size. We need better ways to measure them; that’s another thing we’re working on.
Tell me about your team. How many people are in your lab?

Around 10 people. It’s a good size and a really nice mix of collaborative people from different backgrounds and different parts of the world. The group includes undergraduate students, graduate students, postdocs and junior faculty, and sometimes MD fellows interested in cardiovascular complications of diabetes. And I’m lucky to have several amazing research scientists who often have their own projects in the lab, and keep the wheels turning.
I’ve been very fortunate to have fantastic people in my lab. I couldn’t do any research without this amazing group, and I’m hoping that I contribute to their excitement for science and to their careers.
Was there anything that you learned from your mentors along the way that you apply now as a mentor yourself?
I’ve been extremely lucky to have such great mentors throughout my career. One thing that that Ross and Krebs taught me was to never let yourself be drawn down a path that you think might be right but your experiments show is not. If your experiments are not working, there is a reason, and that reason might be that your preconceived ideas are not correct. You should always keep an open mind and continue to ask questions. You might end up finding something unexpected. That’s key in science, to keep an open mind and to not get stuck in a way of thinking.
Another important part of my personality that my mentors reinforced was to keep trying, never give up. You might not find the answers to your most important research questions for many years, or decades even, but if you just have the determination to get the answer, you will often get there, and you will have advanced science in the process. We have come quite a long way in answering the question of how diabetes leads to an increased risk of cardiovascular disease. But of course, there are ever more questions to be answered. That is exciting to me.
What are your favorite things to do outside of the lab?
I love spending time in nature, birdwatching and taking nature walks. Close to where we live, there is a beaver lodge, right next to the University of Washington campus. It’s so peaceful to see these animals at sunset, only part of their heads showing above the water, chomping on water lilies. Sometimes, the osprey flies over. It’s magical and rejuvenating. I often get new ideas related to the research in my lab during such times.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Thiam elected to EMBO
He was recognized during the EMBO Members’ Meeting in Heidelberg, Germany, in October.

The timekeepers of proteostasis
Learn about the cover of the winter 2026 ASBMB Today issue, illustrated by ASBMB member Megan Mitchem.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

