CSR reviews its reviewers — and makes changes
Noni Byrnes, director of the National Institutes of Health’s Center for Scientific Review, on her Review Matters blog last week, wrote about the need to broaden the pool of NIH reviewers participating in study sections. Byrnes made the case for a more diverse pool of reviewers, saying, “Bringing new viewpoints into the process replenishes and refreshes the study section, enhancing the quality and its output."
CSR took a look at the career stages and frequency of reviewers over the past 12 years and found that some policies enacted to ensure quality review of NIH grants have had the unintended consequence of excluding midcareer and early-career researchers from the review process — and may have induced some reviewers to participate too frequently, giving them disproportionate influence over peer review in their fields.
In her post, Byrnes highlighted CSR guidance that at least 50% of a review committee be full professors. The intention was to ensure that experienced reviewers participated in the process; however, many study sections took this guidance as a directive to prioritize full professors, thus, in some cases blocking midcareer and junior scientists from participating.
Byrnes also reports an upcoming change in policy for frequent reviewers. Currently, reviewers who have “recent substantial service” (defined as serving six or more times in 18 months) are offered continuous submission status as a reward for their service. "This policy, established in 2009 with the best of intentions to incentivize review, has led to the unintended consequence of encouraging excessive ad hoc review service within a short time frame,” she wrote. The CSR Advisory Council unanimously recommended discontinuing that policy, and CSR has outlined its plan for revocation of the rule.
Comments on Byrnes’ blog and social media indicate the community, unsurprisingly, has strong views about study section makeup. Most of those who’ve chimed in seem to agree on the need for these changes and to appreciate the efforts Byrnes outlined. A side debate about a controversial proposal that would require all NIH-funded investigators to participate at least once in peer review also has emerged.
The American Society for Biochemistry and Molecular Biology Public Affairs Advisory Committee frequently discusses (and meets with CSR to discuss) how to improve study sections and guarantee the highest quality of peer review. We would love to hear what you think on this issue.
Do you think the CSR is doing the right thing? What are some policies you think would help improve peer review? How do you feel about mandating participation in peer review?
Email us and tell us what you think!
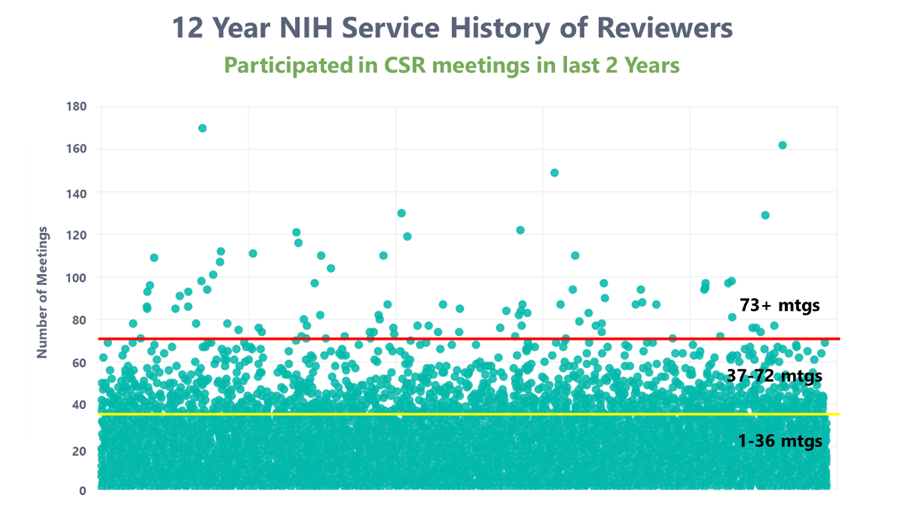
Excerpt from "Broadening the Pool of NIH Reviewers"
"(Above) is a plot examining the service records of all 24,642 reviewers who served as reviewers (in a capacity other than as a mail reviewer) for CSR within the past two years. Each dot represents a reviewer’s number of meetings for the NIH over the last 12 years. Below the yellow line are reviewers who have served one to 36 times in 12 years—these make up 94% of the total pool and include the vast majority of the reviewers used. Between the yellow and red lines are reviewers who have served at 37 to 72 meetings over 12 years, 5% of the pool. The small number of reviewers above the red line have served at 73 or more meetings in 12 years; this is the sort of excessive review service that raises concerns about undue influence."
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Policy
Policy highlights or most popular articles

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Building a stronger future for research funding
Hear from Eric Gascho of the Coalition for Health Funding about federal public health investments, the value of collaboration and how scientists can help shape the future of research funding.

Councilors advocate for science on Capitol Hill
ASBMB Councilors meet with their elected officials to advocate for basic scientific research funding and training the next generation of scientists.

Hope for a cure hangs on research
Amid drastic proposed cuts to biomedical research, rare disease families like Hailey Adkisson’s fight for survival and hope. Without funding, science can’t “catch up” to help the patients who need it most.

Supporting science through advocacy and community building
ASBMB calls on scientists to take action as funding cuts and policy shifts threaten the U.S. research enterprise, emphasizing the power of community advocacy and persistence in protecting the future of science.

Seven steps to advocating in your home state
Find out how to schedule, prepare for and conduct a productive district office meeting to communicate the importance of fundamental scientific research funding to your representatives.

