ASBMB seeks feedback on NIH plan to change grant review criteria, scoring
The American Society for Biochemistry and Molecular Biology is seeking feedback on a National Institutes of Health proposal that would change the way peer reviewers evaluate grant applications. The society’s survey will close Feb. 28.
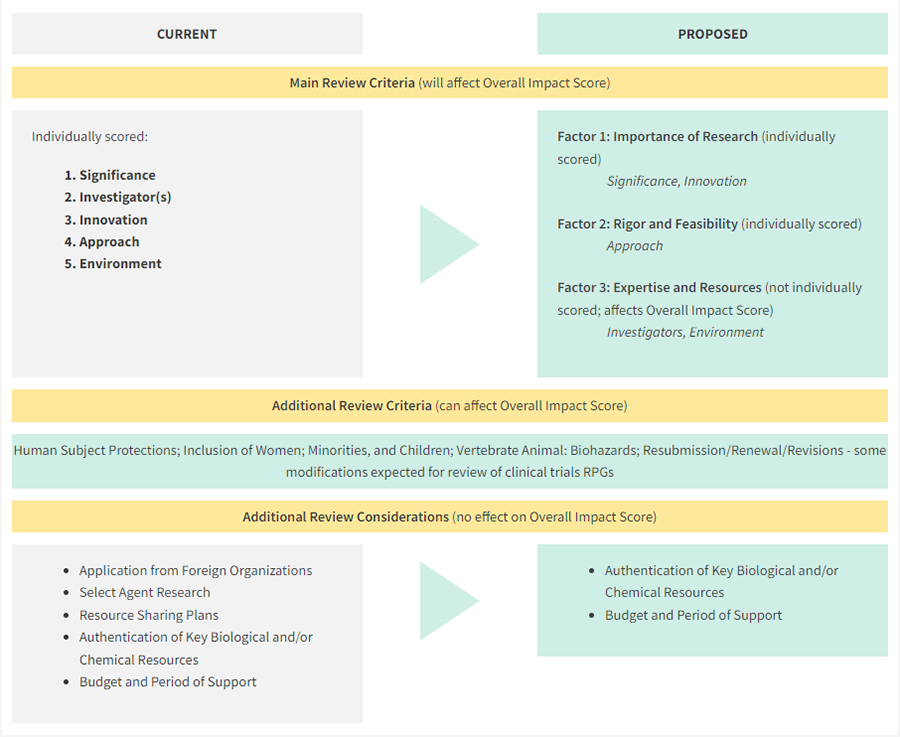
The NIH Center for Scientific Review issued a solicitation in December for public input on a proposed revised framework that would reduce the number of review criteria and change how they are scored.
The goal is two-fold. First, the NIH seeks to emphasize research merit and reduce emphasis on the reputation of the applicant investigators and their institutions. Second, the NIH seeks to reduce the administrative burden on study section volunteers.
Change is needed
The peer-review process ensures that the NIH funds excellent grant applications that will lead to important discoveries.
Reviewers who serve on study sections lend their expertise to evaluate the scientific merit of research proposals. However, some struggle to distinguish between significance and innovation; others have criticized the peer-review process for enabling reputational bias, which disadvantages grant applicants from underrepresented racial groups and low-resourced institutions.
Over the past decade, the peer-review process has become increasingly burdensome on reviewers, who now must also evaluate compliance forms and nonscientific materials.
What’s different?
The CSR Simplifying Review Criteria Workgroup proposes reducing the number of categories reviewers must evaluate and that reviewers focus on two major questions: “Should it be done?” and “Can it be done well?”
In addition, the working group proposes removing individual scoring for the investigator and environment. Instead, reviewers would qualitatively evaluate an applicant’s expertise and resources with ratings ranging from “fully capable” to “additional resources needed.”
If a reviewer finds inadequacies, they would have to justify them in writing. Notably, the applicant would have a chance to rebut concerns raised about expertise and resources.
Reviewers also would be required to provide a written justification for their overall score.
This approach is designed to shift the focus of grant review from investigator and institutional reputation to the merit of the proposed research, thereby reducing positive bias, to align with the CSR’s initiative to reduce bias in peer review.
Furthermore, study section volunteers would review less material from the “Additional Review Considerations” section of the application.
The ASBMB supports the NIH’s efforts to reduce bias in peer review and administrative burden on reviewers.
The public affairs team will collect feedback on the proposed framework through Feb. 28 and incorporate it into the society’s response to the NIH request for information.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Policy
Policy highlights or most popular articles

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Building a stronger future for research funding
Hear from Eric Gascho of the Coalition for Health Funding about federal public health investments, the value of collaboration and how scientists can help shape the future of research funding.

Councilors advocate for science on Capitol Hill
ASBMB Councilors meet with their elected officials to advocate for basic scientific research funding and training the next generation of scientists.

Hope for a cure hangs on research
Amid drastic proposed cuts to biomedical research, rare disease families like Hailey Adkisson’s fight for survival and hope. Without funding, science can’t “catch up” to help the patients who need it most.

Supporting science through advocacy and community building
ASBMB calls on scientists to take action as funding cuts and policy shifts threaten the U.S. research enterprise, emphasizing the power of community advocacy and persistence in protecting the future of science.

Seven steps to advocating in your home state
Find out how to schedule, prepare for and conduct a productive district office meeting to communicate the importance of fundamental scientific research funding to your representatives.