Meet Phyllis Hanson
When our cells come into contact with pathogens, particulates and phagocytosed material, they tend to come out of it the worse for wear, often gaining nanometer-size holes. Were it not for the mysterious work of filamentous ESCRT proteins, these holes might soon spell rupture and death.
These proteins — which drive various membrane remodeling events such as fission reactions that release intralumenal vesicles into endosomes and viruses from the cell surface —are one of Phylllis Hanson’s many research interests.
Hanson earned a bachelor’s degree in biophysics and biochemistry from Yale University in 1985 followed by both an M.D. and a Ph.D. in cancer biology at Stanford University in 1993. She returned to Yale for a postdoctoral fellowship in membrane trafficking before joining Washington University in St. Louis as an assistant professor in 1997.
After two decades researching protein–protein and protein–membrane interactions involved in membrane trafficking at WUSTL, Hanson recently moved her lab to the University of Michigan Medical School’s department of biochemistry — which she now chairs.
Hanson joined the ranks of associate editors at the Journal of Biological Chemistry in November 2017. She spoke with John Arnst, ASBMB Today’s science writer, about her work. The interview has been edited for clarity and length.
What is your group focused on?
My long-term overall interest is in the cell biology of molecular machines, with a particular interest in understanding how proteins regulate the structure and organization of membranes, both inside and outside the cell.
Much of our current work is focused on a set of proteins known as the ESCRT machinery — in particular, the part of that machinery that’s called ESCRT-3. (Author’s note: ESCRT stands for endosomal sorting complexes required for transport.) We’re looking at their role in an increasingly huge range of cell biological processes, and we’re particularly excited right now about a recent discovery that the ESCRT machinery responds to and facilitates the repair of super-small-scale damage in membranes that can be caused by any number of insults from the environment. A big question that we’re trying to address is how the ESCRTs deal with these nano-size disruptions.  During her time at Washington University in St. Louis, Phyllis Hanson helped lead an initiative to establish the Center for Cellular Imaging. She is excited to be growing her new lab at the University of Michigan.Courtesy of PHYLLIS HANSON
During her time at Washington University in St. Louis, Phyllis Hanson helped lead an initiative to establish the Center for Cellular Imaging. She is excited to be growing her new lab at the University of Michigan.Courtesy of PHYLLIS HANSON
What was your academic background and training?
My focus has always been on understanding biochemical mechanisms, but I’ve consistently been drawn to complex puzzles for which there often isn’t a clear road map, and observations from the realm of cell biology have been key for guiding my work. When I did the research part of my M.D./Ph.D., I was working on understanding how the protein CaM kinase II potentiates calcium signaling and, through its autophosphorylation, provides cells and especially neuronal synapses with an important “molecular memory.”
As a postdoc, I wanted to learn more about the nuts and bolts of synapses and moved to study the biochemistry of proteins responsible for synaptic vesicle trafficking. These include SNAREs, which mediate vesicle function, and NSF, a AAA+ ATPase that maintains protein dynamics of the SNAREs, and thus membrane trafficking. Author’s note: SNARE stands for soluble NSF attachment protein receptors, and NSF stands for N-ethlmaleimide sensitive fusion protein.)
When I started my own lab, I continued to work with these but also was drawn to less well-charted problems. I used a set of enzymes, AAA+ ATPases, as my group’s entry point, because I knew how to study them and could see that key members, including the ESCRT-regulatory AAA+ ATPase VPS4 and the dystonia-associated AAA+ ATPase torsinA, were regulating cellular membranes in different and unexpected ways.
From there, we’ve taken big advantage of imaging to help understand what the molecular machinery we study can do, which helps define our problems. One important approach for us is the technique of deep-etch electron microscopy pioneered by my Washington University colleague John Heuser. It involves making platinum replicas of biological material to provide unique three-dimensional information about the spatial relationships between proteins and the membranes they act on; it has really helped piece together the biochemical and cellular puzzles that we’re interested in.
Many years ago, I read a review article about membrane fusion, which I was working on at the time, with a title that raised the ever-present question of mimicry and mechanism in model systems. In other words, how well do the things we study in the test tube mimic the complicated processes in the cell? The significance of this question is that we have to understand biochemical mechanisms but also work to validate and extend what we learn into cells. One of the exciting aspects of today’s biochemistry is that there are new ways to do this and clearly much more on the horizon in what I like to call cellular biochemistry.
Did anything occur in a milestone sort of way that made you choose science as a career?
My father was an experimental physicist, a real tinkerer, and so it was probably both nature and nurture, as it were: I’ve always been fascinated with figuring out how things work.
I also was very much drawn to biomedicine, and I had the good fortune to study separately for a Ph.D. and an M.D. at Stanford. I liked both parts of my training, and the different experiences helped me bring into focus what I really wanted to do. My heart was always in driving the research forward, wanting to answer questions beyond the edge of what was really known. The privilege of having trained alongside truly outstanding clinicians at Stanford makes it easier for me to understand how doctors and other people who are on the front lines of solving health-related problems think about and build on advances in fundamental biomedical science.
What factors were important to your decision to move to Michigan?
It was a combination of things, but I think, really, it was the realization that I want to and can make a difference in helping lead a biochemistry department at a medical school. I cannot emphasize enough how important basic research is to medical schools. Part of why I was drawn to this role is that I think I can both advocate for what we’re doing in a biochemistry department and help find connections between what people in biochemistry can do and medical problems that need solving — as a matchmaker of sorts.
There’s also the caliber of science in my own area at Michigan, with a lot of membrane-trafficking research and an incredible diversity of strong cell biology, biochemistry, biophysics and chemistry spread all across the campus. The number of collaborative interactions that are possible is truly mind-blowing.
And I’m thrilled to be in a department with a storied history. JBC Deputy Editor Fred Guengerich trained here, and several of the JBC AEs have been through this department. I also am joining current Michigan AEs Ruma Banerjee, Eric Fearon and Ursula Jakob.
There’s just a huge energy at Michigan right now, and expanding biological chemistry in this environment is going to be fun.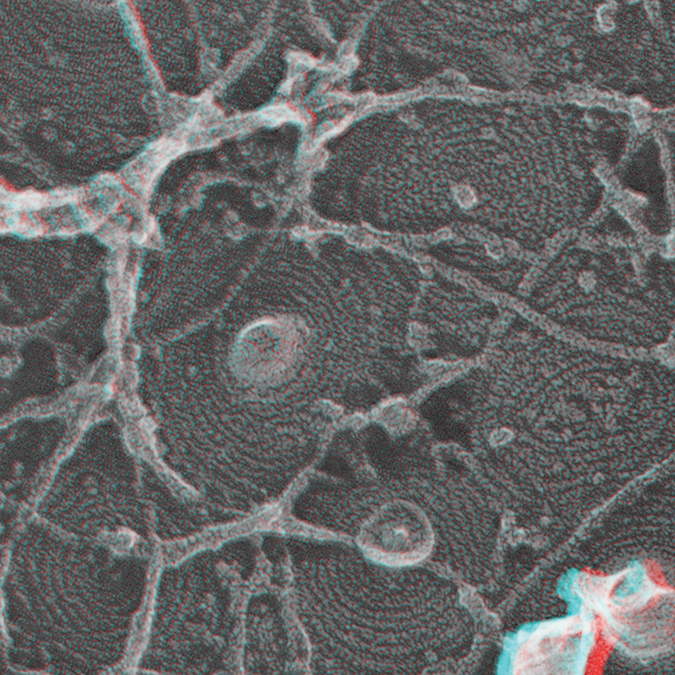 Researchers in Hanson’s lab made stereoimages like this by taking two photos with a slight angle offset from each other and turning them into anaglyphs, where the two pictures are overlaid in two different colors. When viewers don red-green glasses, those colors are sent separately to their eyes, giving them a 3D view of the structure. The box measures 500 nm by 500 nm.courtesy of PHYLLIS HANSON
Researchers in Hanson’s lab made stereoimages like this by taking two photos with a slight angle offset from each other and turning them into anaglyphs, where the two pictures are overlaid in two different colors. When viewers don red-green glasses, those colors are sent separately to their eyes, giving them a 3D view of the structure. The box measures 500 nm by 500 nm.courtesy of PHYLLIS HANSON
When did you first become involved with JBC, and how is your new role so far?
When I was a Ph.D. student at Stanford, my adviser, Howard Schulman, was really dedicated to the JBC, as were a bunch of AEs who were there and leaders at that time. I sent one of my most important papers on inhibitory autophosphorylation of CaMKII to JBC, and I continued submitting papers to JBC as a postdoc and faculty member because I valued its impact and the quality of its editorial board reviews. I have always worked in communities for which JBC has been critical.
It’s great to see so much enthusiasm and selflessness from journal leaders who are doing everything they can to keep pace with the times. That’s an exciting thing to be a part of.
Going forward, I’d like to help expand the reach of JBC in critical areas of membrane trafficking, because these fields are now answering many questions that truly represent biological chemistry.
What do you do outside the lab? Any advice for balancing life in the lab with life outside of it?
I like to spend time with my family. I certainly am a big believer in exercise, for its own sake and for its energy-multiplying effect, and I love to cook.
I believe that there is a close correlation between people who love doing lab work and people who love to cook, especially when you get to being a PI and don’t get to do as much lab work as you used to. That’s a big hobby, and I enjoy cooking especially for entertaining. One of my favorites is paella, both because I remember this as my mom’s go-to crowd dish and because it’s this beautiful thing that you can creatively choose different ingredients to match the occasion at hand.
As far as balancing life, you’ve got to have fun with the science. If you aren’t having fun, then it becomes difficult to manage everything, because you are spending a lot of your time with this stuff. You’ve got to believe in what you’re doing.
Do you have any advice for young scientists?
You have to find the kind of question, or level of question, that works for you. For some people, the pace of experiments matters a lot, and that is something to keep in mind as you choose the approaches that will allow you to satisfy your curiosity, gain traction in a field and contribute to solving puzzles in cellular biochemistry.
And most importantly — enjoy the ride.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Thiam elected to EMBO
He was recognized during the EMBO Members’ Meeting in Heidelberg, Germany, in October.

The timekeepers of proteostasis
Learn about the cover of the winter 2026 ASBMB Today issue, illustrated by ASBMB member Megan Mitchem.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Summer research spotlight
The 2025 Undergraduate Research Award recipients share results and insights from their lab experiences.

