For October, magnesium helps the leaves stay green
We mark the 150th anniversary of Dimitri Mendeleev’s periodic table of chemical elements this year by highlighting elements with fundamental roles in biochemistry and molecular biology. So far, we’ve covered hydrogen, iron, sodium, potassium, chlorine, copper, calcium, phosphorus, carbon, nitrogen, oxygen and manganese.
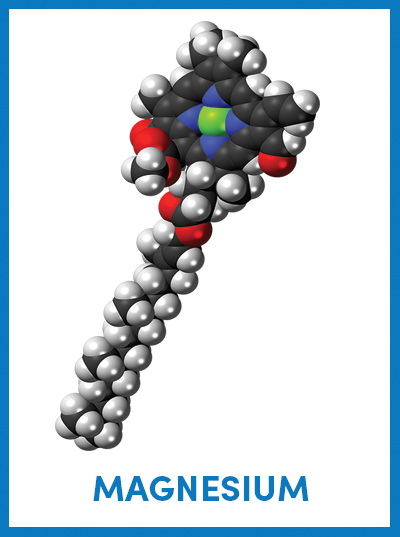 The chemical structure of chlorophyll shows a magnesium ion in green sequestered at the center of a porphyrin ring, which is attached to a long hydrocarbon tail. Jynto/Wikimedia Commons
The chemical structure of chlorophyll shows a magnesium ion in green sequestered at the center of a porphyrin ring, which is attached to a long hydrocarbon tail. Jynto/Wikimedia Commons
October is the month for peak fall foliage in states across the northern and central U.S. Leaves turn yellow, orange and red as chlorophyll — the photosynthetic pigment that gives plants their green color — breaks down due to limited sunlight and cooler temperatures. Chlorophyll consists of a porphyrin ring — a molecule made of carbon, nitrogen and hydrogen — attached to a long hydrocarbon tail. At the center of the porphyrin ring, a magnesium ion stabilizes the chlorophyll molecule and transfers electrons down an electron transport chain to drive the photosynthetic process.
Magnesium — with symbol Mg and atomic number 12 — is a reactive alkaline earth metal that readily can lose two electrons to form a cation with charge +2. In nature, it occurs mostly in compounds with other elements such as carbon, sulfate, oxygen and chlorine.
Magnesium is the ninth most abundant element in the known universe. It is produced in large stars when helium fuses with neon or in supernovas when three atoms of helium fuse sequentially with a carbon nucleus. Supernova explosions disperse magnesium into space, where it falls onto the surface of planets, and into the interstellar medium, where it’s recycled into other star systems.
On Earth, magnesium is the fourth most common element after iron, oxygen and silicon. It is the eighth most abundant element in the Earth’s crust, where it forms large mineral deposits of magnesite, dolomite and other rocks. Magnesium salts easily dissolve in water, making oceans and rivers the most abundant source of biologically available magnesium.
In vertebrates, magnesium is the fourth most common metal ion and the second most abundant intracellular cation after potassium. Protein transporters that carry magnesium across biological membranes must recognize the cation’s large hydration shell and deliver the naked ion. Examples of magnesium transporters are the XntAp protein in the freshwater ciliate Paramecium, the MgtA and MgtB system in the pathogenic bacteria Salmonella, and the SLC41A1 carrier in the plasma membrane of mammals.
Inside the cell, magnesium can be found in the cytosol or stored in intracellular compartments. Free cytosolic Mg+2 alters the cell’s electrical properties by regulating the function of voltage-dependent Ca+2 and K+ channels. This has important consequences in excitable cells such as neurons and muscles, and it regulates processes like neurotransmitter release and muscle contraction and relaxation. Magnesium bound to protein plays a structural role as part of the protein’s conformation or a regulatory role by activating or inhibiting enzyme activity. Magnesium within mitochondria affects the enzymes of energy metabolism and the process of programmed cell death, or apoptosis. And in the nucleus, most of the magnesium is associated with nucleic acids and free nucleotides, neutralizing the negative charge of phosphate groups.
A year of (bio)chemical elements
Read the whole series:
For January, it’s atomic No. 1
For February, it’s iron — atomic No. 26
For March, it’s a renal three-fer: sodium, potassium and chlorine
For April, it’s copper — atomic No. 29
For May, it’s in your bones: calcium and phosphorus
For June and July, it’s atomic Nos. 6 and 7
Breathe deep — for August, it’s oxygen
Manganese seldom travels alone
For October, magnesium helps the leaves stay green
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

