An ever-growing role for a tiny lipid
Critical signaling functions have been attributed to phosphatidic acid, or PA, the smallest building block in phospholipid biosynthesis. With a small head group and a net negative charge ranging from -1 to -2 depending on pH, PA can modulate local membrane geometry and recruit a large set of specific proteins to confined membrane subdomains; a recent review by Emeline Tanguy and colleagues summarizes both of these essential actions for PA’s signaling function. PA is versatile and challenging because it can be produced and metabolized by a large set of enzymes.
Although PA is present at low levels in most cell types, it appears to be critical for neuronal and glial cell function. Several neurological diseases maybe attributed, at least in part, to altered PA synthesis and/or catabolism. For example, Ricardos Tabet and colleagues proposed in 2016 that an alteration of the PA/diacylglycerol balance could be a main cause of fragile X syndrome, a genetic cause of intellectual disability. They showed that diacylglycerol kinase-kappa, or DGK-kappa, mRNA was the main target of fragile X mental retardation protein and that reduced DGK-kappa expression impaired PA synthesis in neurons from mice bred not to express FMR1, a protein that male fragile X patients lack. Silencing DGK-kappa in pyramidal neurons from the CA1 region of the hippocampus largely reproduced fragile X symptoms. This PA/DAG imbalance is thus likely to affect DAG and PA downstream signaling required for both maturation of dendritic spines and establishment of correct synaptic plasticity.
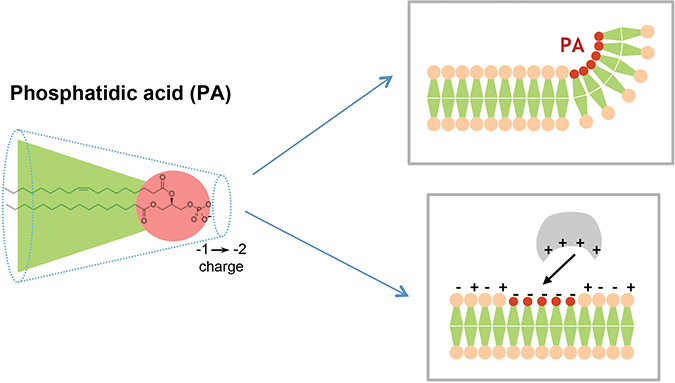 Phosphatidic acid is defined by its shape and charge. This conical lipid generates negative membrane curvature and is negatively charged, thereby recruiting positively charged proteins. Nicolas Vitale
Phosphatidic acid is defined by its shape and charge. This conical lipid generates negative membrane curvature and is negatively charged, thereby recruiting positively charged proteins. Nicolas Vitale
Maria Zeniou–Meyer and colleagues in 2008 proposed that the loss of expression of the kinase RSK2, which leads to Coffin–Lowry syndrome (a rare syndromic form of mental retardation), reduced PA synthesis and distorted neurosecretion. Increased PA synthesis has been reported in gliobastoma, the most frequent and aggressive brain cancer. A link between brain PA levels and Alzheimer’s disease also is starting to emerge, but the exact effects of PA imbalance in neurodegeneration and cognitive deficits has not been identified. Finally, reduced PA synthesis may contribute to fetal alcohol spectrum disorders, as ethanol leads to phospholipase D-mediated phosphatidylethanol production at the expense of PA.
Many cellular pleiotropic functions of PA rely on its ability to regulate actin cytoskeleton dynamics and to modulate membrane-involved functions. The former occurs mainly through PA’s ability to modulate the activity of small GTPases, including Rho and Arf members. The latter probably results both from the original cone shape structure of PA favoring negative membrane curvature and from PA’s net negative charge allowing local recruitment of specific proteins. One remaining challenge is to define precisely sites of PA synthesis within the brain and at the subcellular level in neurons. Recent improvements in lipidomics allow for sensitive quantification of dozens of PA species made with different fatty acids. According to a study by Nawal Kassas and colleagues, the development of novel genetically encoded PA sensors also will be crucial in localizing and potentially quantifying changes in PA and perhaps different PA species.
Despite PA’s low abundance, its relative simplicity, and the complexity of its metabolic and signaling pathways, improved understanding of its multiple functions in brain development and function is now within reach.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
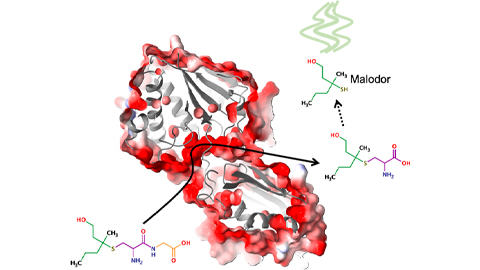
Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.

Neurobiology of stress and substance use
MOSAIC scholar and proud Latino, Bryan Cruz of Scripps Research Institute studies the neurochemical origins of PTSD-related alcohol use using a multidisciplinary approach.
Pesticide disrupts neuronal potentiation
New research reveals how deltamethrin may disrupt brain development by altering the protein cargo of brain-derived extracellular vesicles. Read more about this recent Molecular & Cellular Proteomics article.

A look into the rice glycoproteome
Researchers mapped posttranslational modifications in Oryza sativa, revealing hundreds of alterations tied to key plant processes. Read more about this recent Molecular & Cellular Proteomics paper.

Proteomic variation in heart tissues
By tracking protein changes in stem cell–derived heart cells, researchers from Cedars-Sinai uncovered surprising diversity — including a potential new cell type — that could reshape how we study and treat heart disease.

Parsing plant pigment pathways
Erich Grotewold of Michigan State University, an ASBMB Breakthroughs speaker, discusses his work on the genetic regulation of flavonoid biosynthesis.

