Chronic pain after trauma may depend on your stress gene
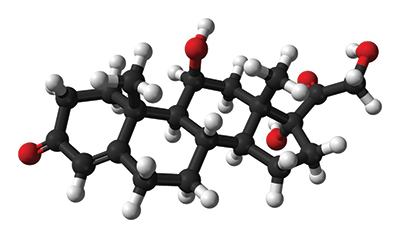 This ball-and-stick model represents a molecule of cortisol, known as the “stress hormone.” Cortisol and adrenaline have been shown to sensitize peripheral nerves directly, which enables cortisol to signal pain in the absence of nerve injury. Public domain
This ball-and-stick model represents a molecule of cortisol, known as the “stress hormone.” Cortisol and adrenaline have been shown to sensitize peripheral nerves directly, which enables cortisol to signal pain in the absence of nerve injury. Public domain
Almost every person in the world will experience at least one traumatic event, such as a car crash, an assault, war combat or a natural disaster, during their lifetime. Many will endure more than one.
Most people recover from a traumatic incident, but a substantial percentage develop chronic problems, including post-traumatic stress symptoms, depression and chronic pain.
Chronic pain? Isn’t pain caused by nerve injury? Not always. Chronic pain can develop and is quitecommon after trauma exposure. This might seem surprising given the fact that many traumas involve little or no tissue damage.
I am a geneticist and molecular biologist studying predictors and mediators of chronic pain and other chronic neuropsychiatric conditions that develop after a traumatic experience. I am particularly interested in understanding the biological reasons why some individuals are more vulnerable to chronic pain than others.
Based on previous findings from our research group and others, my colleagues and I hypothesized that individual genetic variation affects who develops pain and who recovers after trauma exposure. To test this hypothesis, our group at the Institute for Trauma Recovery, led by Samuel McLean, enrolled individuals in a study of European-Americans and African-Americans who had been involved in a traumatic motor vehicle collision. We collected blood samples from more than 1,500 such individuals and assessed their DNA and their pain levels six weeks after the car crash.
Trauma, then pain
Before I go into details about this study, let’s brainstorm how chronic pain might develop after trauma. If we know how pain develops, we can find treatments to prevent its onset. And by preventing the onset of chronic pain, we eliminate the need to use addictive and potentially deadly opioids.
Exposure to traumatic events causes our stress system to activate. This stress system sends signals between the hypothalamus in the brain, the pituitary gland and the adrenal gland, ultimately resulting in the release of cortisol, commonly known as the “stress hormone.”
Cortisol is a critical link between trauma and chronic pain. This is because cortisol and another stress hormone called adrenaline have been shown to sensitize peripheral nerves directly, which enables cortisol to signal pain in the absence of nerve injury. For this reason, it is vital for our bodies to regulate cortisol levels carefully and to resolve the stress response quickly and effectively.
Regulating the stress hormone
Our bodies have natural regulators of blood cortisol levels. Typically, a protein called the glucocorticoid receptor, or GR, binds to cortisol that is released after stress exposure and causes cells to alter activities of the immune system and brain. But another protein called FKBP5 also can manipulate cortisol levels by binding GR and preventing it from binding cortisol.
If FKBP5 levels are high, that sequesters the GR and prevents the GR from binding and lowering blood cortisol levels. Consequently, levels of cortisol in the blood can rise and potentially cause harm by binding nerve endings and causing pain. Previousstudies have shown that a person’s genes can influence relative levels of these proteins.
Based on this knowledge, our group hypothesized that the ability of FKBP5 to regulate cortisol and potentially affect pain levels might originate in our DNA. We tested this hypothesis using data from our more than 1,500 car crash survivors.
Importantly, these individuals experienced trauma but did not have bone fractures or tissue injury.
We chose motor vehicle collision as our trauma exposure because it is common and highly traumatic, and we can capture data in the immediate aftermath of the traumatic incident. Physicians in emergency departments across the country helped us enroll individuals and collect blood from them so that we could measure DNA, RNA, microRNA and hormone levels. This was important because for this study we wanted to understand how all of these types of molecules are related and how their composition can vary from one individual to the next.
Our pain and our genes
Our group discovered that which genetic variant of the FKBP5 gene a person carries is predictive of how much post-traumatic chronic pain that individual will experience following motor vehicle collision.
Our study of car crash survivors showed that rare variants TG and GG in the stress response gene FKBP5 increase vulnerability to developing chronic pain.
Both African-American and European-American individuals carrying at least one copy of the less common variants FKBP5-TG or FKBP5-GG experienced more pain than the individuals carrying only the more common FKBP5-TT variant. (We all have two copies of every chromosome, so we can carry two different versions or variants of the same gene.)
We then wanted to know how these variations affect the stress response and subsequent chronic pain.
We knew that individuals with the less common variants FKBP5-TG or FKBP5-GG are more likely to experience pain after trauma exposure. We predicted that in these individuals, FKBP5 regulation of cortisol would be abnormal. We measured cortisol in these individuals and found that their cortisol levels were higher with respect to FKBP5 levels than the cortisol levels of individuals carrying the FKBP5-TT variant, who have less pain.
The common TT gene variant causes less cortisol to build up in the blood and less pain. The rare TG and GG variants cause cortisol levels to surge, and this can trigger chronic pain.
The effect on microRNA binding
We wanted to find the molecular mechanism through which this genetic variant alters FKBP5 and related cortisol levels. Our first clue was that the gene variant we were studying is located in the 3ˈuntranslated region, or UTR, of the FKBP5 messenger RNA. The 3ˈUTR contains many regulatory elements that control the amount of protein produced from a gene. One such type of regulatory element is a microRNA binding site.
microRNAs bind to mRNA, usually in the 3ˈUTR region, and silence the bound genetic message. Humans have more than 2,000 different microRNAs, which bind to mRNA through specific base pairing. Our group and others have shown that microRNAs such as miR-320a and miR-15 can bind FKBP5. Therefore, we examined the genetic sequence surrounding the FKBP5 gene variant we had shown to be associated with post-traumatic chronic pain and found a predicted miR-320a site just over 100 nucleotides upstream of the variant.
With more than 100 nucleotides separating the genetic variant and the potential miR-320a binding site, it was clear that the genetic variant did not influence microRNA binding directly in the same way seen in a previous study. However, in collaboration with Alain Laederach’s group at the University of North Carolina at Chapel Hill, we determined that the genetic variant influenced the ability of the FKBP5 RNA to fold into a secondary structure.
The exact folding of the RNA secondary structure differed depending on whether the FKBP5 gene contained the TT variant or the TG/GG variant. Using RNA structural modeling called SHAPE, we showed that the RNA structure varied significantly in the region of the miR-320a binding site, such that miR-320a could bind in the presence of the protective variant but could not bind efficiently in the presence of the risk variant.
Therefore, in people with the TG or GG genetic variants, the variant causes the FKBP5 RNA to fold into a secondary structure that inhibits miR-320a silencing of FKBP5. This disinhibition leads to increased FKBP5 levels and increased post-traumatic chronic pain.
Overall, this recent discovery by our group suggests a way that humans can develop chronic pain following trauma exposure without experiencing tissue injury. It also highlights a gene involved in the development of post-traumatic chronic pain that could be a promising new target for drug therapies. And it proposes a mechanism through which this gene is regulated naturally.
This last point can help us in our quest to discover specific types of therapeutics, because, for instance, if we didn’t want to try to target FKBP5 directly, we could mimic the action of this naturally occurring regulatory mechanism. Our work suggests that with such a potential therapeutic, we could preferentially treat individuals with the DNA variant that causes more pain.
A version of this article was published in The Conversation website under a Creative Commons license. Read the original article.
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Iron could be key to treating a global parasitic disease
A study has found that leishmaniasis causes body-wide changes in iron balance, leading to red blood cell damage.

Environmental DNA is everywhere
The ability to extract trace bits of DNA from soil, water, and even air is revolutionizing science. Are there pitfalls?
Early COVID-19 research is riddled with poor methods and low-quality results
The pandemic worsened, but didn’t create, this problem for science.

From the journals: MCP
Three views of mass spec: analyzing secreted protein spectra, imaging mass spectrometry for clinical use and spectral libraries for MS data analysis. Read about these recent papers.

Understanding the fat science
Researchers at UCLA investigate lipid remodeling in the liver for energy generation.

No oxygen? No problem
By studying how electric fish survive in hypoxic streams for months at time, researchers may find new ways to target tumors.


