Speedy treatment against drug-resistant bacteria
A new technique my colleagues and I developed that can kill deadly, multidrug-resistant bacteria in real time could be used to generate targeted therapies that replace traditional, increasingly ineffective antibiotics.
Bacteria follow the same basic genetic process that all organisms do: DNA, which contains instructions on how an organism will look and function, is copied into an intermediate form called RNA that can be translated into proteins and other molecules the organism can use.
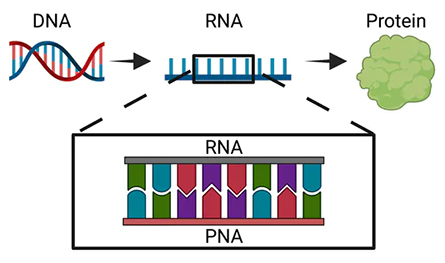
into protein or other useful biological molecules necessary for life.
The technique we developed at the Chatterjee Lab at the University of Colorado Boulder uses a synthetic version of RNA called PNA, or peptide nucleic acid, to disrupt this basic process in bacteria. Our PNA molecule clings to the bacterial RNA, blocking it from carrying out its job. Because this molecule is a perfect match to bacterial RNA, it binds very tightly to the RNA and resists degradation. This means that it can not only escape the bacteria’s error detection processes but also prevent that RNA from being translated into proteins and other useful biological molecules. This impediment can be lethal to the bacteria.
Our study, which we recently published in Communications Biology, demonstrates the therapeutic potential of a technique that can design, synthesize and test PNA treatments in under a week.
Most antibiotics aren’t specific enough to target only infectious bacteria without also destroying the body’s good bacteria. Our technology, however, uses noninfectious versions of multidrug-resistant bacteria to create highly specific molecules. By targeting just the pathogen of interest, these PNA therapeutics may avoid the harm that current antibiotics pose to the body’s good bacteria.
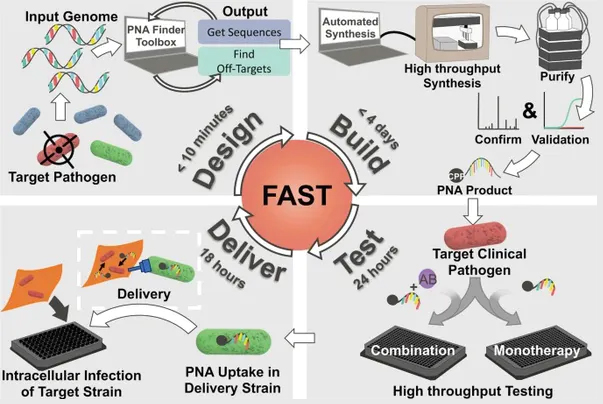
bacteria in under a week.
Why it matters
Bacteria’s adaptation to survive current antibiotics, or antibiotic resistance, is on the rise.
Medicine’s current arsenal of treatments mostly consist of naturally occurring antibiotics that were isolated more than 30 years ago. Discovery of new antibiotics in nature has stagnated while bacteria continue to evolve and evade current treatments. And even if scientists were to find a new natural antibiotic, research shows that bacteria will begin to develop resistance within as little as 10 years, leaving us in the same predicament as before.
New types of therapies need to be considered for a post-antibiotic era, a time when our arsenal of antibiotics is no longer effective. By using a system that can target specific bacteria and be continuously modified based on emerging resistance patterns, doctors would no longer have to rely on chance discoveries. Treatments can adapt with bacteria.
What still isn’t known
Although we explore multiple characteristics that determine which RNA sequences are the best targets, more research is necessary to identify the most effective PNA therapeutics against multidrug-resistant bacteria. As our study only tested our new strategy on cell cultures in the lab, we’ll also need to see how it works in living animals to maximize the effectiveness of this kind of treatment.
What’s next
Our team is currently testing the technology in different animal models against different types of infections. We are also exploring other PNA delivery options, including adapting our bacterial delivery system to probiotic strains so it can integrate with the existing healthy bacteria population in the body.
With further development, our goal is to adapt the platform to target diseases that also use the same basic genetic processes as bacteria, such as viral infections or cancer.
The Research Brief is a short take about interesting academic work.
This article is republished from The Conversation under a Creative Commons license. Read the original article.
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

Fasting, fat and the molecular switches that keep us alive
Nutritional biochemist and JLR AE Sander Kersten has spent decades uncovering how the body adapts to fasting. His discoveries on lipid metabolism and gene regulation reveal how our ancient survival mechanisms may hold keys to modern metabolic health.

Redefining excellence to drive equity and innovation
Donita Brady will receive the ASBMB Ruth Kirschstein Award for Maximizing Access in Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Mining microbes for rare earth solutions
Joseph Cotruvo, Jr., will receive the ASBMB Mildred Cohn Young Investigator Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Fueling healthier aging, connecting metabolism stress and time
Biochemist Melanie McReynolds investigates how metabolism and stress shape the aging process. Her research on NAD+, a molecule central to cellular energy, reveals how maintaining its balance could promote healthier, longer lives.

Mapping proteins, one side chain at a time
Roland Dunbrack Jr. will receive the ASBMB DeLano Award for Computational Biosciences at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

