
“The magic isn’t the squid …
The magic is the protein.”
Just offshore from Redondo Beach in Los Angeles, there’s an underwater canyon. According to local scuba enthusiasts, once a year it becomes a highway to an invertebrate orgy in the shallows.
Underwater photographer–videographer Brent Durand and others in the Los Angeles scuba diving community keep an eye out each December for signs of the squid run, when the footlong animals emerge en masse from the canyon into the shallow water near shore to mate.

“The squid are just filling the entire water column,” Durand said. In some places near the bottom, divers find themselves “surrounded by a wall of squid so dense that if you reach to grab one of your gauges, you might have a squid between your hand and the gauge.
“You have to be OK with squid being pressed against your face.”
Squids are intensely visual animals, and the swarm is attracted by the divers’ lights. According to Durand, it is easy to tell whether another dive group has passed through recently by how many are already amassed when he descends to the bottom. For him, while the throngs are impressive, the moments when he’s near fewer animals can be even more compelling.
“On the outside of the mating aggregation, you can really quiet down,” Durand said. Sometimes a single squid “will hover in front of you, and you feel like you’re almost connecting, checking each other out.”
At those moments, watching the squid’s rapidly changing color displays, a diver may glimpse something unique to the California market squid and a few of its cousins: their tunable iridescence. The phenomenon is a remarkable feat of optics not known to happen in other biological contexts. New research in the Journal of Biological Chemistry sheds some light onto the biochemistry that makes it work.
How is iridescence generated?
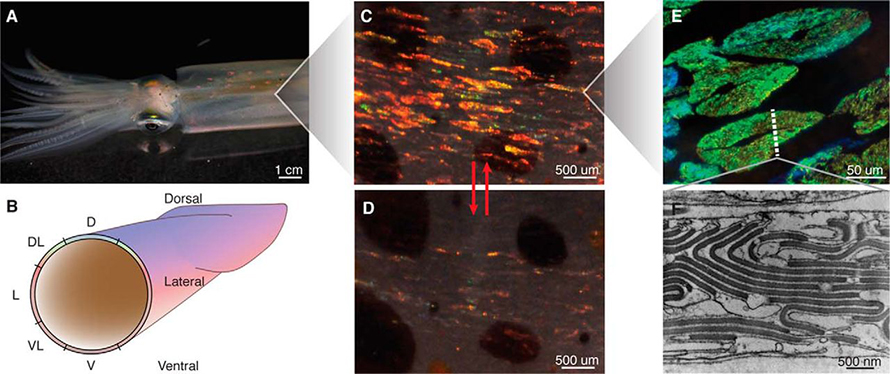
Most cephalopods can change color swiftly through a small palette of pigmentary yellows, reds and browns — an ability that makes them stars of many “now you see me, now you don’t” camouflage videos. This is controlled by chromatophores, cells with a sac of pigment that expand or contract with muscle activity. In a small family of shallow-water squid species that includes the California market squid, the Caribbean reef squid, and a few other flashy cousins, the palette is nearly unlimited.
On these squids’ mantles, when the chromatophores are retracted, they reveal splashes of color arranged in a pattern that looks a bit like leopard skin. Each bright splotch is made up of a few dozen to a few hundred cells called iridocytes. Unlike chromatophores, iridocytes use structural color. Pigment produces a color by absorbing certain wavelengths and reflecting others that eventually reach the observer’s eye. But structural color depends on a different optical phenomenon, which occurs when light reflects from a regular, repeated structure.

It’s a phenomenon called Bragg reflection. Dan Morse, a distinguished professor at the University of California, Santa Barbara, explained: “An example is the rainbow of reflection that you can see from the surface of a CD disk. The distance between the grooves determines the color, the wavelength, at which constructive reflection occurs.”
Squid iridocytes have just such a structure. “The iridophore cells contain a stack of membrane-enclosed sandwiches of protein that are very thin, something like a stack of Frisbees within the cell,” Morse said. “The light scattering is coming from the interfaces between the membrane and the external space.”
Whether light is passing through layers of plastic and air, as in the CD, or of cell contents and extracellular fluid, as in the squid, whenever a large number of alternating layers with different refractive indices are repeated at the same distance apart, some light can be reflected off each layer. For light whose wavelength is about four times the distance between layers, those reflections will be in phase, allowing constructive interference between rays. Other wavelengths will not get this brightness boost.
In most animals with structural coloration, such as iridescent beetles, bright butterflies or even certain types of cuttlefish and octopus, the reflectors’ dimensions are fixed, keeping the reflection at one wavelength — or, in the case of iridescence, a few wavelengths that differ according to the observer’s angle. But squids in the loliginid family can alter the structure of the tiny features inside iridophores, changing the color that results — or even whether iridescent patches appear on their milky skin at all.
Dynamic iridescence depends on the brain
For a long time, researchers were stumped about how squids could turn their iridescence on and off.

A colleague and friendly collaborator/competitor of Morse, Roger Hanlon of the Marine Biological Laboratory in Woods Hole, Massachusetts (now an affiliate of the University of Chicago), was part of a team that reported in the late 1980s and early 1990s that iridophores’ change from colorless to iridescent came with a change in thickness of the protein-dense plates within iridocytes and could be stimulated by applying the neurotransmitter acetylcholine.
Among neuroscientists who study vertebrates, acetylcholine is most familiar for its role in the neuromuscular junction (though cholinergic cells also are found throughout the brain). It is responsible for the signaling between neurons in the peripheral nervous system and the muscle cells that do those neurons’ bidding.
The same light-scattering properties that make iridophores so bright also made them difficult to study using light microscopy. Scientists could not see whether neurons were forming synapses at iridophores; stimulating various nerves did not seem to cause the iridescence to change. And as cephalopod researchers had learned in one physiological system after another, to expect squid systems to behave just like human systems was to be disappointed by eons of divergent evolution.
“These goofy animals are built so differently (from us),” Hanlon said. “They’re doing things that aren’t by the book — and they’re sure not by the vertebrate books.”
So he considered it possible that, like a starfish’s tube feet, iridophores might be responding to acetylcholine that diffused in from another tissue. But there were problems with that hypothesis, too — mostly the speed at which iridophores change compared with sluggish starfish.

As the squids get visual information, Hanlon said, “The animal has to integrate that information ‘upstairs’ and it has to make all the motor output — that being the millions chromatophores and iridophores — they have to be turned on, and it all happens in about one to one and a half seconds.”
It was a puzzle until, in 2012, Hanlon’s lab reported that the delivery of acetylcholine to control color was, indeed, neuronal. In a follow-up paper, they worked out the unusual neuroanatomy that had so confused their previous investigations.
Protein phase separation drives color change
In the meantime, the researchers had made some progress on how acetylcholine could alter the spacing of light reflectors within iridophores. The accordionlike ruffled-membrane stacks are packed with a protein called reflectin. A 2009 collaboration between Morse and Hanlon’s labs had found that reflectin is phosphorylated upon acetylcholine stimulation. Morse’s recent research shows exactly how that phosphorylation drives color change.
“This very complex, multistep process is largely contained in reflectin,” Morse said.
Morse’s lab showed in 2013 that the distance between the stacks was determined by water efflux. At rest, an intact iridocyte’s membrane-folded plates are swollen with water. Using deuterated water, the researchers showed that the cell will expel that water after treatment with acetylcholine.
“Dehydration shrinks the thickness and spacing of the membrane-enclosed layers, changing the wavelength that’s reflected,” Morse said. The more water is expelled, the shorter the distance between stacks and the shorter the wavelength that will be reflected best.
Since 2013, research from Morse’s lab, chronicled in a series of papers in the Journal of Biological Chemistry, gradually has elaborated a biochemical mechanism linking water expulsion with acetylcholine signaling. The color change is driven by inducible phase separation that happens after reflectin is phosphorylated.
Reflectin proteins are conserved among many cephalopod species. There are four isoforms in the California market squid. The relectin that fills and determines the distance between alternating layers in the iridocyte has an alternating structure of its own. Between conserved domains, it has linker regions rich in arginine and aromatic amino residues. These domains have a strong net positive charge and do not lend themselves to secondary structure.
According to Morse, reflectin’s two types of domains have opposing activities. “The cationic linkers are like compression-resisting shock absorbers. Because of electrostatic repulsion, they want to stretch out and keep the protein in an extended, unstructured form. The alternating domains are like the spring on our screen doors: They want to compress, but they’re held in check by the repulsion of the linkers.”
When acetylcholine is released, it activates a tyrosine kinase — the enzyme’s exact identity is not yet known — that phosphorylates the linker regions. Adding negatively charged phosphates neutralizes the positive charge of those domains, reducing their repulsion and letting reflectin assemblies begin to form. With more phosphorylation, the positive charge eventually is neutralized, allowing formation of larger complexes.
“As the reflectin particles become fewer and bigger, osmotic pressure within the stacks drives more and more liquid out,” Morse said. “Now, the contents are very dense by this time … which means that the opacity increases. But the color of that reflectance is set by the dimensions separating the membrane layers.”
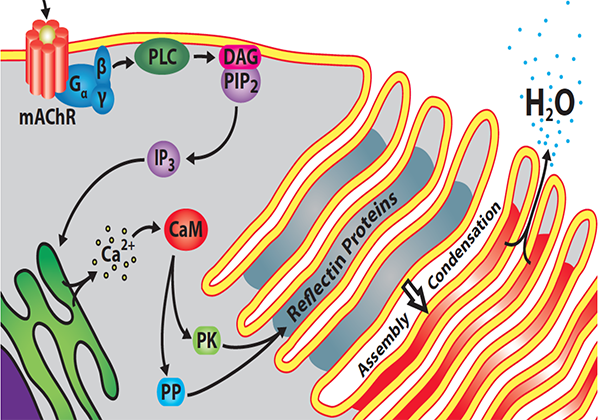
While acetylcholine activates the kinase in question, Morse said that the phosphatase seems to be constitutively active. “The result is that the signal that activates iridescence is ephemeral. From moment to moment, there’s a pulse of neuronal signal in response to whatever the animal sees. The brain fires this neuron; acetylcholine is released and diffuses to these cells, activating the color change; but then the phosphates quickly are removed. That means the system produces a color in response to a neuronal signal and then can be reset very quickly.
“You can see the tissue return to colorless if you provide no further stimulus. But if stimulus is continuing and changing, then the color can continuously change.”
Morse’s work has garnered attention for his meticulous attention to the biophysics of reflectin’s response to signaling. Hanlon said, “I think Dan’s avenue of inquiry, looking more and more carefully at very specific mechanisms of subtle changes in nanostructure and in how the reflectin protein changes its conformation is really going to be important to understand the system.”
Engineering materials based on reflectin
As reflectin has come into focus, researchers have begun considering ways to use the protein, which Morse often calls a “marvelous molecular machine,” to inspire bioengineering efforts.
“Because of its domains’ opposing physical activities, the protein works as a sensor that can measure signals and produce a proportional calibrated output,” Morse said. “Can we transport this very precisely calibrated transduction … into other signal-dependent, proportionally switching reconfigurable materials? If so, we could design reconfigurable materials that might be used for many applications.”
For example, he said, the ability to control the viscosity of bulk quantities of protein might be used to solve problems in protein manufacturing for pharmaceutical or industrial purposes.
Meanwhile, researchers led by materials scientist Alon Gorodetsky at the University of California, Irvine, are working on developing stimulus-responsive biomaterials inspired by reflectin.
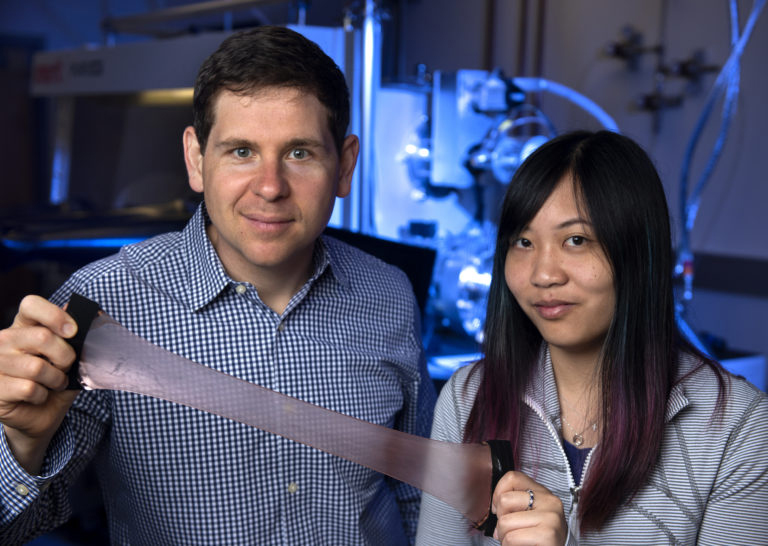
It combines layers transparent to infrared light with adjustable layers that reflect infrared light, and is responsive to stretching.
“The first day I was starting my independent position, I walked into a room where Roger Hanlon was giving a talk about cephalopods’ camouflage ability,” Gorodetsky said. “It was just mindblowing. I said, ‘OK, I’m dropping half the research that I was planning on doing and switching to reflectin.’”
According to Gorodetsky, reflectin’s combination of properties is unique. “I can’t think of another protein that has this high refractive index and excellent electrical properties, can be processed so easily and is also very stable.”
It’s stable enough, in fact, that his lab developed a tape coated in a thin film of reflectin that changes infrared reflectivity after being stretched. On the other hand, it is so responsive to many stimuli — pH, ionic strength, humidity, charge — that it is not an ideal material for reusable technologies. Instead, Gorodetsky said, his lab now uses the protein as inspiration to derive materials that improve upon its qualities.
Back in the world of basic science, Morse is also curious about whether the reversible assembly of reflectin could shed light someday on other types of protein assembly — such as the formation of amyloid plaques in the brain that occurs early in Alzheimer’s disease. “Both classes of proteins, the amyloids and the reflectins, have extended domains of positively charged amino acids that appear to form liquid–liquid phase separated droplet intermediates,” Morse said. “It raises the question: Why is this process reversible, harmless and even advantageous in the case of reflectin, but irreversible and pathological in the case of Alzheimer’s?”
Plenty also remains to be learned about reflectin’s function in the squid. Gorodetsky said that one of the most interesting questions is how different reflectin isoforms, which have high sequence identity, create different optical effects in different cells.
Ask Morse about his favorite aspects of his work, and the answer is the same. “Its elucidation of this complex molecular machine embodied in the reflectin protein. The magic isn’t the squid. The magic isn't the photonics. The magic is the protein machine and its mechanism of action.”
Colorful, yet colorblind

Researchers don’t know exactly why squid use tunable iridescence. According to Lydia Mäthger, a scientist at the Marine Biological Laboratory in Woods Hole, Massachusetts, “There isn’t anything that really proves that any of this is used for communication between the squid.”
But, Mäthger added, it is difficult to study ocean animals’ behavior and very difficult to prove a hypothesis about intraspecific signaling. “Say you did film something that was definitely a signal. Then the question still is, did the neighboring squid that looked at it perceive it as a signal? And what does it mean?”
In her first paper as a graduate student, published in 2001, Mäthger investigated how iridescence might look underwater. She recalls her adviser telling her to imagine the squid 30 meters below the surface and think about how their skin might actually appear at that depth.
Using data collected by divers, Mäthger did just that. At the depths the squid frequent, sunlight is filtered to blue-green by the water above, so it is unlikely that the animals enjoy the color displays they are famous for. That’s doubly certain since cephalopods express just one photoreceptor, well suited to blue-green light, and cannot differentiate between wavelengths as humans do.
“When one of these iridophores reflects red under white light,” Mäthger explained, “for the squid, red is just a very dim green. If an iridophore reflects green, that’ll be a really bright green at the depths at which they occur.”
Another feature of marine light is that nearly all of it comes straight down. That enabled Mäthger to predict exactly how an activated iridescent patch would appear to an observer above, below or alongside the animal. Her results suggested that the iridescence may help both with camouflage and schooling. To an observer looking straight down from above, the squid will be scarcely visible. To an observer at the same depth, it will appear about four times as bright as the background.
Subsequent research has suggested other potential explanations for tunable iridescence. Among male loliginid squid, a flash of iridescence is part of aggressive displays. Among females, some researchers have argued that when two stripes of iridescence flanking a white stripe show up, that may be a signal that they are not receptive to mating. However, no one has proved the function of any visual signal conclusively.
Still, Mäthger said, given the small size of the field and the squids’ reliance on light, “It would be kind of silly to assume, only because it’s not been proved scientifically, that they don’t use it.”
— Laurel Oldach

Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
How a gene spurs tooth development
University of Iowa researchers find a clue in a rare genetic disorder’s missing chromosome.
New class of antimicrobials discovered in soil bacteria
Scientists have mined Streptomyces for antibiotics for nearly a century, but the newly identified umbrella toxin escaped notice.
New study finds potential targets at chromosome ends for degenerative disease prevention
UC Santa Cruz inventors of nanopore sequencing hail innovative use of their revolutionary genetic-reading technique.
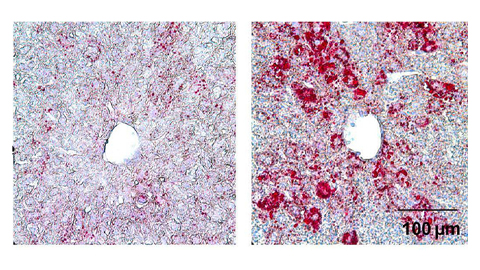
From the journals: JLR
How lipogenesis works in liver steatosis. Removing protein aggregates from stressed cells. Linking plasma lipid profiles to cardiovascular health. Read about recent papers on these topics.
Small protein plays a big role in viral battles
Nef, an HIV accessory protein, manipulates protein expression in extracellular vesicles, leading to improved understanding of HIV-1 pathogenesis.

Genetics studies have a diversity problem that researchers struggle to fix
Researchers in South Carolina are trying to build a DNA database to better understand how genetics affects health risks. But they’re struggling to recruit enough Black participants.

