FDA approves CRISPR–Cas9 therapy for sickle cell disease
Officials at the U.S. Food and Drug Administration today announced approval of the first CRISPR–Cas9 therapeutic to treat sickle cell disease. The cell therapy is the only cure to date for patients who are not eligible for stem cell transplants. The FDA approved the therapy for patients age 12 to 35.
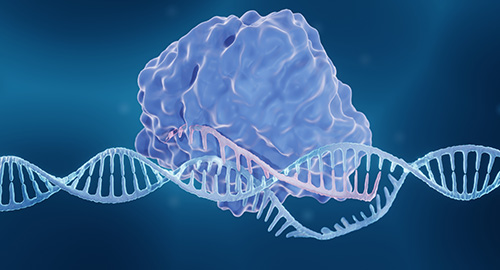
The therapeutic, exagamglogene autotemcel, sold as Casgevy, from CRISPR Therapeutics and Vertex Pharmaceuticals, uses CRISPR–Cas9 technology to edit a patient’s own hematopoietic stem cells to produce high levels of fetal hemoglobin, which is not normally expressed in adulthood and drowns out the damaging effects of their sickle hemoglobin. The therapy was approved in the United Kingdom in November.
Sickle cell disease is a debilitating, genetic disorder that causes abnormal red blood cell development. It affects approximately 7.74 million individuals worldwide. Red blood cells are normally disk-shaped and move easily throughout the blood, carrying oxygen to vital organs. In a patient with sickle cell disease, red blood cells are warped, or “sickle”-shaped, which can lead to impaired blood flow and cause stroke, infection, eye issues, severe pain crises and premature death.
The therapeutic specifically targets BCL11A, a repressor of the fetal hemoglobin gene, using a guide RNA. The precise target site is a residue within the BCL11A enhancer region, which, once modified by the Cas9 nuclease, takes the brakes off the fetal hemoglobin gene, leaving transcription and later translation free to occur.
Victoria Gray, a mother of four from Mississippi, was the first sickle cell patient to receive Casgevy in a 2019 clinical trial. Prior to the treatment, Gray said she had to rush to the emergency room at least once a month and often stay in the hospital for weeks at a time due to her painful crises. Since receiving Casgevy, she has had no emergency room visits, hospital stays or crises, she said.
“I feel cured,” Gray said. “My life has changed dramatically with just a leap of faith.”

Emmanuelle Charpentier is a cofounder of CRISPR Therapeutics and the director of and a scientific member at the Max Planck Unit for the Science of Pathogens. Charpentier shared the 2020 Nobel Prize in chemistry for the development of CRISPR–Cas9 with Jennifer Doudna, an RNA biochemist at the University of California, Berkeley.
Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, commented on today's action. “These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” Marks said. “Today’s actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDA’s commitment to facilitating development of safe and effective treatments for conditions with severe impacts on human health.”
The FDA approved Casgevy based on three studies showing that the drug is a safe and effective treatment for sickle cell disease. In the most recent safety and efficacy clinical trial, patients like Gray who received the therapeutic sustained long-term high levels of total hemoglobin, similar to what is seen in healthy adults, and most were blood transfusion–independent and free of painful crises for at least one year after treatment.
Benjamin Oakes, CEO and cofounder of Scribe Therapeutics, said “The approval is just the tip of the iceberg. It's a really beautiful proof of concept for what CRISPR genome editing can do. … The real vision and mission for the whole CRISPR field should be to create safer, more effective therapies that can be brought more broadly to patients.”

Oakes said he and his colleagues are developing the next generation of CRISPR gene therapies using an engineered CasX protein that cuts DNA more precisely than Cas9.
According to the Institute for Clinical and Economic Review, Casgevy could be priced at up to $1.93 million to be cost-effective, a measure that estimates how much it costs to gain a unit of a health outcome, such as a life year gained or a death prevented. With this price tag, ICER estimated that 15% of eligible patients could be treated over a five-year period.
In 2022, patients with sickle cell disease incurred out-of-pocket medical costs totaling up to $44,000, with insurers covering approximately $1.7 million per patient. Economists predict that gene therapies like Casgevy could cost a single state Medicare program more than $30 million per year.
The FDA will evaluate a second gene therapy for sickle cell disease, bluebird bio’s lovo-cel, later this month. Unlike Casgevy, lovo-cel uses a lentiviral vector to introduce a modified beta globin gene into patient stem cells. This modified gene produces an antisickling hemoglobin protein, which is designed to inhibit the polymerization of mutant sickle hemoglobin, making it less likely to form blockages in the circulation.
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

What’s next in the Ozempic era
Diabetes, weight loss and now heart health: A new family of drugs is changing the way scientists are thinking about obesity — and more uses are on the horizon.
How a gene spurs tooth development
University of Iowa researchers find a clue in a rare genetic disorder’s missing chromosome.
New class of antimicrobials discovered in soil bacteria
Scientists have mined Streptomyces for antibiotics for nearly a century, but the newly identified umbrella toxin escaped notice.
New study finds potential targets at chromosome ends for degenerative disease prevention
UC Santa Cruz inventors of nanopore sequencing hail innovative use of their revolutionary genetic-reading technique.
From the journals: JLR
How lipogenesis works in liver steatosis. Removing protein aggregates from stressed cells. Linking plasma lipid profiles to cardiovascular health. Read about recent papers on these topics.
Small protein plays a big role in viral battles
Nef, an HIV accessory protein, manipulates protein expression in extracellular vesicles, leading to improved understanding of HIV-1 pathogenesis.

