MCP: Keeping tabs on protein variants
Perhaps you have seen a time-lapse video of a busy city sidewalk. As people come and go, they blur together into a crowd with no distinguishing features. You could count the number of people pushing strollers in each frame, but it might be hard to tell how long one parent has been circling the same block with a colicky baby.

As proteins are made and destroyed in a cell, they tend to blur together too. Many proteomics studies measure with precision the number of copies of each protein species but not how long each one lasts. In a new paper in the journal Molecular & Cellular Proteomics, researchers in Bernard Kuster’s lab at the Technical University of Munich report a new approach to determining the lifespan of a great many proteins, and their alternative isoforms, in large data sets.
“Plenty of research has demonstrated that cancer, neurodegenerative diseases, age-related diseases and even aging per se are associated with altered lifespans of single proteins or a global dysregulation of the cellular recycling machinery,” said lead author Jana Zecha. She compares a cell in which proteins are continuously made and destroyed to “a tiny protein production and recycling machinery.” With colleagues, Zecha set out to measure this factory’s output, determining the rates of production and destruction of many different proteins.
The researchers combined two techniques for telling samples apart by their mass: stable isotope labeling by amino acids in cell culture, or SILAC for short, and tandem mass tag labeling, or TMT. The primary SILAC label enabled a pulse-chase experiment, a way of measuring how much of a new amino acid is taken up after it is added to cells. By combining SILAC with TMT, the researchers could achieve high proteome coverage with high reproducibility and accurate counts of each protein. Then they looked for trends over time. For example, a protein’s rate of synthesis can be measured by how much of the new SILAC label appears over time in its spectrum, and degradation is measured by how much the old label disappears.
Other scientists previously had combined the SILAC and TMT methods, but this data set gave an unusually thorough look at protein lifetimes. The researchers found substantial variability among splice variants of proteins, which no one had yet measured in a data set of this size. Because two splice variants from the same gene have many peptides in common, a data set with many measurements at the peptide level was required.
The approach could offer a better way of understanding the basic biology of disease states with altered protein turnover. The researchers also are interested in modifications occurring after translation that may alter turnover rates.
“A proteomewide measurement of turnover rates of modified peptides is the next logical step for us,” Zecha said.
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Immune cells can adapt to invading pathogens
A team of bioengineers studies how T cells decide whether to fight now or prepare for the next battle.
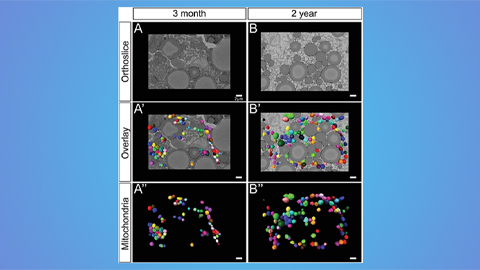
Hinton lab maps structure of mitochondria at different life stages
An international team determines the differences in the 3D morphology of mitochondria and cristae, their inner membrane folds, in brown adipose tissue.

National Academies propose initiative to sequence all RNA molecules
Unlocking the epitranscriptome could transform health, medicine, agriculture, energy and national security.
From the journals: JLR
What can you do with artificial lipoproteins? A new key to angiogenesis. Flavonoids counteract oxidative stress. Read about recent papers on these topics.
Iron could be key to treating a global parasitic disease
A study has found that leishmaniasis causes body-wide changes in iron balance, leading to red blood cell damage.

Environmental DNA is everywhere
The ability to extract trace bits of DNA from soil, water, and even air is revolutionizing science. Are there pitfalls?

