From the journals: April 2018
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
A chaperone-client relationship in cancer cells
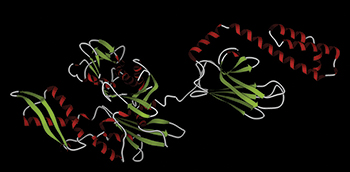 In this illustration of Hsp70, the alpha helices are shown in red, and beta strands are shown in green.Courtesy of Meng0987/Wikimedia CommonsMany aggressive tumors have an overabundance of the chaperone Hsp70, which is thought to stabilize proteins that promote tumor growth. Until recently, not much was known about the identities of Hsp70’s clients, hindering efforts to develop cancer drugs targeting Hsp70.
In this illustration of Hsp70, the alpha helices are shown in red, and beta strands are shown in green.Courtesy of Meng0987/Wikimedia CommonsMany aggressive tumors have an overabundance of the chaperone Hsp70, which is thought to stabilize proteins that promote tumor growth. Until recently, not much was known about the identities of Hsp70’s clients, hindering efforts to develop cancer drugs targeting Hsp70.
New research from labs at the University of California, San Francisco, and the University of Michigan identifies some of these clients. The results were published in the Journal of Biological Chemistry.
According to Jason Gestwicki, the professor at UCSF who oversaw the study, “It has been known for many years that Hsp70 is one of the major proteins required (for rapid cell division in tumors). Yet chemical inhibitors of Hsp70 have not advanced to clinical trials. One of the stumbling blocks is that it is difficult to tell if Hsp70 has been inhibited in a cell.”
The new study, led by then-graduate student Laura Cesa, demonstrates that Hsp70 stabilizes two proteins that inhibit apoptosis, or programmed cell death, in breast cancer cell lines. Importantly, one of these (X-linked inhibitor of apoptosis protein, known as XIAP) is uniquely stabilized by Hsp70, not the better-studied chaperone Hsp90.
“Now that we know that XIAP levels decrease in the presence of Hsp70 inhibitors, we can use XIAP levels as a way to estimate how much Hsp70 has been inhibited,” Gestwicki said. “This knowledge will allow us to more rapidly create improved Hsp70 inhibitors as potential anti-cancer agents.”
— Sasha Mushegian
A new weapon in bacterial warfare
Bacterial type VI secretion systems, or T6SSs, through which some bacteria can inject toxins into others, are recognized to be important for interbacterial competition, including in the context of the plant microbiome that protects plants from pathogens. The identities of the toxic effectors delivered through these systems are often unknown. Jenny Tang, Nathan Bullen and colleagues at McMaster University write in the Journal of Biological Chemistry that they discovered that the T6SS effector of the plant-protective bacterium Pseudomonas protegens is an NAD(P)+-degrading enzyme. Searching other bacterial genomes suggested that NADases are found in many other bacterial species with type VI and type VII secretion systems, hinting that these enzymes may be widespread weapons in bacterial competition, with consequences for plant and human health.
Why FGFR inhibitor therapy may fail
Fibroblast growth factor receptors, called FGFRs for short, activate many cell-growth pathways and are overactive in some types of cancer. Although FGFR inhibitors seem like a promising possible drug class, they have failed in clinical trials because they sometimes, puzzlingly, cannot stop the growth of cancers with high FGFR activity. A study published in Molecular & Cellular Proteomics has revealed a new regulator of at least one FGFR, and that finding may help explain why treatment frequently fails. Researchers supervised by Jørgen Wesche at the Norwegian Radium Hospital in Oslo found a new FGFR1 regulator, the protein tyrosine phosphatase receptor PTPRG. PTPRG is required to shut down FGFR signaling. When PTPRG is absent, osteosarcoma cells more effectively resist treatment with an FGFR1 inhibitor. The authors suggest that the effectiveness of many FGFR inhibitors may depend on whether PTPRG is present in a cancer. If they are correct, clinicians may be better able to predict whether an individual patient would benefit from the drugs.
Making heads or tails of sperm
Acephalic spermatozoa syndrome is a rare genetic condition wherein sperm are produced as decapitated flagella or tailless heads because of defects in the head-tail coupling apparatus. Yonglian Shang and colleagues at the Chinese Academy of Sciences examined the effects of disease-associated mutations in SUN5, a testis-specific nuclear envelope protein, on sperm function. In a paper in the Journal of Biological Chemistry, they write that they found these mutations resulted in SUN5 proteins that were unable to interact with a specific heat shock protein. They hypothesize that the newly discovered interactor is a chaperone that helps SUN5 fold and localize correctly in the “neck” of developing sperm.
When swapping sugar for ketones doesn’t work out
At present, there are 15 studies on clinicaltrials.gov recruiting or preparing to recruit cancer patients to test the ketogenic diet as a therapy. This diet, high in fat and very low in carbohydrates, is said to induce a fasting state without the actual fast. It dramatically lowers the amount of glucose in circulation and increases fatty ketone bodies. The change in energy source is proposed to cut off the sugar supply to metabolically active cancer cells. But preclinical results are surprisingly mixed. A paper in the Journal of Lipid Research explained some of the variability observed in models of ketogenic therapy for cancer. The researchers, led by Jie Zhang and Ping-Ping Jia of Huai’an Hospital in Jiangsu, China, found that some types of cancer are unlikely to respond well to the diet because their cells are well prepared to use ketone bodies as an energy source instead of glucose. The researchers identified four key ketolytic enzymes and found that when the isoforms BDH1 and OXCT1 were expressed at high levels in a cancer cell line, feeding mice with a tumor of those cells a ketogenic diet would in fact speed tumor growth. Conversely, mice whose tumors had lower expression of the enzymes saw slower tumor growth. This research has important implications for when it is appropriate to recommend a ketogenic diet to cancer patients.
Fat balance matters for mouse health
 COURTESY OF WELLCOME IMAGES/WIKIMEDIA COMMONS
COURTESY OF WELLCOME IMAGES/WIKIMEDIA COMMONS
Polyunsaturated fats are the “good fats” according to diet magazines and clean-living advocates. But some unsaturated fats are better than others. In a recent study in the Journal of Lipid Research, a team led by Rayane Ghandour at the Universite Cote d’Azur in France, in collaboration with nutritionists from Germany, compared the effects of diets supplemented with two types of unsaturated fatty acid on formation of brown adipose tissue in mice. Brown fat heats the body and can dissipate excess energy; converting white fat tissue, which is metabolically inert, into brown fat is a hypothetical target for weight-loss treatment. Ghandour tested the effect of supplementing a mouse’s diet with either omega-3 essential fatty acids, which are abundant in fish, or omega-6 fatty acids, which are high in some vegetable oils and soy. High omega-6 intake is known to correlate with inflammation, impaired brown fat formation, and obesity. Although both groups of mice ate the same amount of calories and weighed the same, they responded differently to a treatment that stimulated brown fat tissue production. The mice with omega-3-supplemented feed showed mildly greater weight loss and better ability to convert white adipocytes to brown adipocytes when stimulated than those with omega-6 supplementation. Just one lipid mediator of inflammation, the prostaglandin PGF2-alpha, was significantly different between mice on the two diets. PGF2-alpha is made by an enzyme from omega-6 fatty acids. The researchers tested the enzyme’s activity in cultured cells with omega-6 or both types of fatty acid and found that omega-3 could out-compete omega-6 fatty acids for enzyme activity. This research provides a mechanism for why diets high in omega-6 fatty acids may cause inflammation.
— Laurel Oldach
A redox off switch for a celiac-related protein
Transglutaminase 2, or TG2, is an enzyme involved in the pathogenesis of celiac disease; patients produce autoantibodies against TG2 in complex with dietary gluten. TG2 is post-translationally regulated by a redox switch consisting of a disulfide bond that must be broken by thioredoxin in order to activate the enzyme. In a paper in the Journal of Biological Chemistry, Michael Yi and colleagues at Stanford University identify isomerase ERp57 as the factor that inactivates TG2, thus demonstrating the first example of a reversible disulfide bond switch modulated by two different proteins. The discovery could lead to treatments for celiac disease that target TG2, rather than requiring patients to stay on a lifelong gluten-free diet.
A Western blot sans antibodies
Although antibodies are widely used reagents for semiquantitative measurement of proteins, many are prone to false positives and lot-to-lot variability. A paper in Molecular & Cellular Proteomics offers an alternative that may be more reliable. A research team at the Max Planck Institute of Molecular Cell Biology and Genetics developed a geLC-tandem mass spectrometry workflow adapted to calculate absolute molar quantities of several target proteins in a single run. Lead author Mukesh Kumar and colleagues designed chimera proteins that combined signature peptides from a large number of target proteins and expressed them in E. coli in the media with heavy isotope-labeled amino acids. Gel bands of a chimera protein, target proteins and reference protein (e.g., albumin) were co-digested and analyzed by liquid chromatography and tandem mass spectrometry. Characteristic MS peaks from the labeled chimera peptides could be compared to the same peaks from unlabeled target proteins. Since the chimeric proteins include peptides from a reference protein of known concentration, they can be used to determine a precise quantity of target proteins in the sample. The technique offers a powerful antibody-free tool for analysis of untagged proteins at very low concentrations, which could improve reproducibility in future biochemistry studies.
Better prepping of phosphoproteins
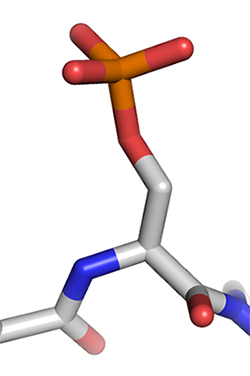 This stick diagram represents a phosphorylated serine residue.Courtesy of Thomas Splettstoesser/Wikimedia Commons
This stick diagram represents a phosphorylated serine residue.Courtesy of Thomas Splettstoesser/Wikimedia Commons
Phosphorylation is a simple, reversible modification that can change protein function completely. Because of phosphorylation’s role in the regulation of nearly every cellular process, it is of great interest to researchers. However, phosphoproteins make up a very small subset of the proteome, making enrichment of phosphorylated proteins mandatory for phosphoproteomics. Immobilized metal-affinity chromatography can be used to enrich phosphoproteins in a sample prior to mass spectrometry due to the high affinity of positively charged metal ions in the column to negatively charged phosphate groups. However, phosphorylated proteins are not the only molecules that can bind to the column. In a recent article in Molecular & Cellular Proteomics, researchers in Simone Lemeer’s group at Utrecht University in the Netherlands identified ions from nucleic acid contaminants that bound to an immobilized iron ion column, making MS analysis more difficult. The same compounds were identified in several published works, confirming that nucleic acid contamination is a widespread problem. The team developed a robust protein sample cleanup using a combination of protein precipitation and nuclease treatment to remove contaminants. As a result, the researchers, led by Clement Potel, identified more than 50 percent more phosphorylation sites in humans and 10-fold in bacteria. This work opens the way for the study of phosphorylation dynamics in microorganisms, which may help further our understanding of signaling in pathogens.
A localization signal for insulinM
Signal peptides are short protein sequences, characterized by particular structural features that direct translocation of secretory proteins into the endoplasmic reticulum for folding and maturation. Most mammalian secretory proteins are translocated co-translationally, but some, including the insulin precursor preproinsulin, are not transported into the ER until translation is complete. In a study in the Journal of Biological Chemistry, Huan Guo and colleagues at Tianjin Medical University found that the signal peptides of post-translationally translocated secretory proteins contain a positive charge that enables translocation and that a diabetes-associated mutation in preproinsulin affects this charge and thus preproinsulin transport. Mutations in this region may contribute, therefore, to hereditary forms of diabetes.
More or less, same enzyme effect
High levels of the lipid ceramide are believed to be a stress trigger for autophagy. When the lysosomal enzyme acid sphingomyelinase, or ASM, which makes ceramide by breaking down a more complex lipid, is overexpressed, the self-digestion process is increased. So you’d expect that inhibiting ASM should reduce autophagy. Scientists at Indiana University found, however, that this was not the case. In a recent study in the Journal of Lipid Research, the team, led by Matthew Justice, found that when ASM was inhibited, the lysosomal nutrient sensing complex turned off, and signaling by a pro-autophagy transcription factor began. This may have been because inhibiting ASM did not lower levels of its product, ceramide, as would be expected; instead, ceramide production rose. The researchers did observe a decrease in the level of a related lipid, sphingosine. They speculated that this may be because of interactions between ASM and an enzyme that produces sphingosine. However, the mechanisms by which ASM affects lysosomal nutrient sensing and sphingosine level remain to be determined. This research sheds light on how cells decide whether to keep growing or turn on the brakes.
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Immune cells can adapt to invading pathogens
A team of bioengineers studies how T cells decide whether to fight now or prepare for the next battle.
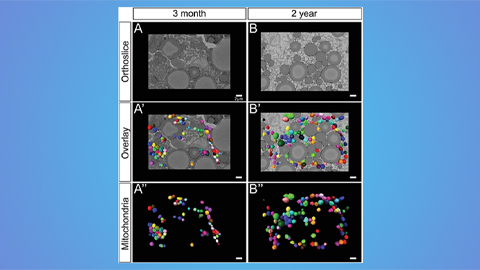
Hinton lab maps structure of mitochondria at different life stages
An international team determines the differences in the 3D morphology of mitochondria and cristae, their inner membrane folds, in brown adipose tissue.

National Academies propose initiative to sequence all RNA molecules
Unlocking the epitranscriptome could transform health, medicine, agriculture, energy and national security.
From the journals: JLR
What can you do with artificial lipoproteins? A new key to angiogenesis. Flavonoids counteract oxidative stress. Read about recent papers on these topics.
Iron could be key to treating a global parasitic disease
A study has found that leishmaniasis causes body-wide changes in iron balance, leading to red blood cell damage.

Environmental DNA is everywhere
The ability to extract trace bits of DNA from soil, water, and even air is revolutionizing science. Are there pitfalls?

.jpg?lang=en-US&width=300&height=300&ext=.jpg)
