The circadian coupling of cellular and solar clocks
Circadian rhythm is a molecular dance that runs on a roughly 24-hour clock — slightly longer and shorter in some organisms —affecting a vast number of physiological systems. Long before scientists uncovered the process in human cells, people observed it in the movement of plants’ leaves.

Engraved portrait of French astronomer and geophysicist Jean-Jacques d’Ortous de Mairan (1678-1771) by Simon Charles Miger (1736-1828). D’Ortous de Mairan noticed that the leaves of a mimosa plant continued to open and close in sync with daylight hours, even when the plant was kept in darkness.courtesy of Wikimedia Commons If you have a plant on your windowsill, it likely expands its leaves during the daylight hours and contracts them at night. It’s less likely that you’ve shoved your plant in a dark closet and checked in on it throughout the day, but if you had you’d have noticed that it keeps up this behavior, caused by its circadian rhythm, even in the absence of sunlight.
The earliest written description of circadian rhythm dates to around 400 BC; a Macedonian ship’s captain named Androsthenes noted that the leaves of tamarind trees open toward the sun during daylight hours and close up at dusk. This diurnal behavior was further explored more than 2,000 years later when French scientist Jean-Jacques d’Ortous de Mairan noted in 1729 that the leaves of the mimosa open and close in sync with daylight even when the plant is kept in darkness, suggesting an endogenous, or internal, factor is at play.
This internal factor, a feedback loop that coordinates a number of cellular processes in a 24-hour period, has been identified and studied extensively in a number of model organisms, including fruit flies, mice and humans. It is believed to be present in various permutations in nearly every organism on Earth. Over the past two decades, researchers the world over have continued to probe the feedback loop that maintains the circadian rhythm and the cycle’s effects on numerous aspects of human physiology, including wound healing, cardioprotection, chemotherapy and pharmacology.
This year, the Nobel Committee awarded its prize for physiology or medicine to American chronobiologists Michael W. Young, Jeffrey C. Hall and Michael Rosbash for their seminal work in the 1980s and ’90s uncovering the molecular mechanisms that drive the circadian rhythm in Drosophila melanogaster, or fruit flies.
According to Frank A.J.L. Scheer, a professor at Harvard Medical School and the director of the Medical Chronobiology Program at Brigham and Women’s Hospital, the laureates’ work put the circadian field on the map. Their research “gave a lot of recognition to the field of circadian biology,” Scheer said. “It made it very concrete. It allowed researchers to develop tools to study cause and effect to manipulate particular core clock molecules.”
The process was slow going at first. After Hall, Rosbash and Young isolated and cloned the first gene that was found to be essential for circadian rhythm in 1984, its function remained unclear for a number of years.
“Animals are rhythmic organisms, and that was something that became apparent as we learned the pieces of this machine by collecting genes, but it certainly wasn’t in our heads at the beginning,” said Young. “Our three small labs got things rolling many years ago, but what’s been very pleasing is how it’s been amplified by findings in so many different systems now.”
A brief history of cellular time
After scientists in the 18th and 19th centuries observed that a variety of organisms continued to display 24-hour behavior when kept in darkness, the geneticist Seymour Benzer and his student Ronald Konopka in 1971 used mutagenesis studies in fruit flies to identify a causative gene, which they named “period.”
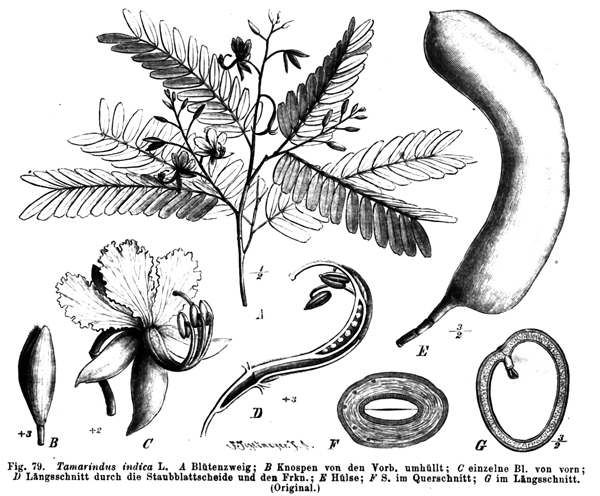 The tamarind plant, illustrated here, was documented as displaying circadian behavior about 2,400 years ago.courtesy of NATÜRLICHE PFLANZENFAMILIEN. VOL. III, 3 (1891)
The tamarind plant, illustrated here, was documented as displaying circadian behavior about 2,400 years ago.courtesy of NATÜRLICHE PFLANZENFAMILIEN. VOL. III, 3 (1891)
This gene codes for a protein, PER, that maintains the circadian rhythm by a negative feedback loop in which the protein accumulates in an organism’s cellular cytoplasm at night and moves to the nucleus shortly before the organism wakes up, where it inhibits its own synthesis. The protein then degrades in both the nucleus and cytoplasm. Similar 24-hour feedback loops have been found in several species of plants and fungi and are believed to be present in all eukaryotes, despite variations in the structures of the responsible genes and proteins.
Steve Kay, a chronobiologist at the University of Southern California, is a longtime collaborator with Hall. “Even though the names of the proteins and even the homology of the proteins might be different across the kingdoms of the eukaryotes, the fundamental core mechanism appears to be very similar,” Kay said.
 Michael W. Young is the Richard and Jeanne Fisher professor and vice president for academic affairs at the Rockefeller University.courtesy of MARIO MORGADO
Michael W. Young is the Richard and Jeanne Fisher professor and vice president for academic affairs at the Rockefeller University.courtesy of MARIO MORGADO
Although it uses a markedly different set of proteins, a version of the circadian rhythm has even been observed in prokaryotes, which eukaryotes diverged from billions of years ago. “We know for sure that clocks have evolved independently multiple times, because it’s clear that the clocks that we find in prokaryotes, notably the clock that was defined in cyanobacteria, is absolutely different from the clock we find in eukaryotes,” Kay said.
In humans, the levels of PER have profound effects on heart rate, blood pressure, metabolism, body temperature and hormone levels. As such, the circadian rhythm is responsible for a number of physiological changes throughout the day — a halt in secretion of the hormone melatonin shortly before waking up; bowel movements and high alertness in mid-morning; subsequent peaks in coordination, reaction time, cardiovascular efficiency and muscular strength throughout the afternoon; and the onset of melatonin secretion in the evening, leading to sleep.
After Hall and Rosbash at Brandeis University and Young at Rockefeller University isolated and cloned the period gene from fruit flies in 1984 and, shortly thereafter, isolated PER, the researchers quickly ran into a problem when it came to deducing the protein’s function based on its structure.
“Sequencing the period gene’s informational content led to an on-paper protein that is large, 1,200 amino acids, but it was totally featureless,” Hall said. “This molecular identification of the gene and then characterization of the gene took the whole enterprise out into terra incognita. We had identified a completely novel protein that nobody had ever seen before.”
The three research groups worked to gradually define the structure and function of the PER protein, as well as search for other genes responsible for the circadian rhythm. Hall and Rosbash discovered in 1988 that PER’s level fluctuates throughout the night and early morning. In 1998, after discovering rhythms in period mRNA levels that were shortened, lengthened or deleted depending on mutations to the period allele, they proposed that an inhibitory feedback loop allowed the PER protein to control the cycling of the period gene’s expression. In 1992, Hall and Rosbash found that this feedback loop is transcriptionally regulated, implying that PER regulates its own transcription and possibly that of other genes that control physiology and behavior.
In 1994, Young’s laboratory found a missing piece of the feedback loop: a second gene, timeless, that coded for a protein, TIM (pronounced “time”). This protein binds to PER proteins in the cytoplasm and ferries them into to the nucleus, where they block the period gene from further synthesizing PER proteins. A few years after discovering TIM, Young’s lab found another protein, DOUBLETIME, which phosphorylates the remaining PER proteins to mark them for degradation.
 Amita Sehgal is currently investigating sleep pathways in fruit flies at the University of Pennsylvania’s Perelman School of Medicine. COURTESY OF AMITA SEGHAL
Amita Sehgal is currently investigating sleep pathways in fruit flies at the University of Pennsylvania’s Perelman School of Medicine. COURTESY OF AMITA SEGHAL
A postdoctoral fellow in Young’s lab, Amita Sehgal, now at the University of Pennsylvania, cloned the tim gene mutant in 1995. Soon thereafter, Sehgal started her own lab at the University of Pennsylvania and she and Young continued to collaborate to clone the tim gene. Subsequently, the Sehgal lab discovered that light degrades TIM, which causes the circadian rhythm to adjust after external conditions, such as what time of day the sun is in the sky, have changed.
John Ewer, a chronobiologist at the University of Valparaiso in Chile, joined Hall’s lab at Brandeis in 1985 as a graduate student. “If you go to Tokyo tomorrow, you’ll wake up at the time of wherever you started off from, and then, little by little, your time will shift,” he said. “That’s a reflection of the fact that the clock is running in you in a phase that is imposed by the light-dark cycle where you started.”
This phase, during which the circadian rhythm affects certain processes, will remain constant throughout an organism’s life if not externally altered. This has been observed in fruit flies, which will display the same phase if consistently exposed to 24-hour light cycles as larvae and then raised in darkness through adulthood without the clock’s activity being reset by light exposure.
Circadian and sleep
If you’ve ever traveled across multiple time zones, you likely felt groggy for a number of days. This phenomenon, jetlag, is caused by a temporary mismatch between your sleep cycle and circadian rhythm.
 Jeffrey C. Hall lives in Cambridge, Maine, and is a professor emeritus of biology at Brandeis University.COURTESY OF MIKE LOVETT
Jeffrey C. Hall lives in Cambridge, Maine, and is a professor emeritus of biology at Brandeis University.COURTESY OF MIKE LOVETT
While circadian rhythm exhibits an effect on sleep cycles, including secretion of melatonin, the two act by separate pathways. In a preindustrial world, the pathways largely would have operated in sync, but the advent of electrical lighting and shift work have combined to disrupt them for a significant portion of the population.
“If you’re up all night, and sleep in the morning, your (circadian) clock is telling you to wake up, and at that point your sleep system has to suppress the circadian-induced activity to allow you to sleep,” Sehgal said. This circadian misalignment has been linked to higher blood pressure, an increase in inflammatory markers and cholesterol levels, and a decrease in insulin sensitivity and glucose tolerance.
Despite the interaction between the pathways for sleep and the circadian rhythm, research involving each occupies distinct fields, Sehgal said. She and her collaborators have developed a Drosophila model for sleep, and are investigating the neurocircuitry of sleep pathways in fruit flies. “The sleep field is, I would say, 15 years behind the circadian field,” she said. “We’re starting to get a framework for circuits, but not necessarily what’s happening during sleep.”
Body clocks in medicine
While the mechanisms by which the clocks in every organ and cell communicate with one another to stay synchronized are still being puzzled out, researchers are investigating aspects of physiology, such as the breakdown of blood clots, wound healing and the morning peak of cardiovascular risk factors, that are under circadian influence.
In a study published in November in the journal Science Translational Medicine, scientists studying fibroblasts, the skin cells essential for healing, in mice, found that proteins that direct the cells’ activity are more active at night, when the mice are active. The researchers found that when the mice were injured during normal waking hours, the wounds healed significantly faster than those that occurred in the hours that mice would normally be sleeping.
 Much like those of the mimosa and tamarind plants, the leaves of the silk plant open during the day and close during the night.courtesy of WIKIMEDIA COMMONS
Much like those of the mimosa and tamarind plants, the leaves of the silk plant open during the day and close during the night.courtesy of WIKIMEDIA COMMONS
Proteins with levels affected by the circadian rhythm also play a role in protecting cardiac cells from damage suffered during low-oxygen conditions, or hypoxia. Following up on observations that people who have heart attacks in the morning tended to fare worse than people who have them in the afternoon, scientists at the University of Lille in France reported in The Lancet in October that using antagonist molecules to decrease the signaling of a circadian rhythm–linked protein, Reverb alpha, decreased the rate of hypoxia-related damage to mouse cardiac cells that were damaged close to the time that they woke up.
Despite being nocturnal, mice displayed a similar pattern of increased cardiac injury as the humans and had more persistent damage when injured closer to the time that they woke up. By comparing the transcriptomes of human patients who underwent heart surgery in the morning and afternoon with the transcriptomes of mice with heart cells damaged during the sleep-to-wake transition and the wake-to-sleep transition, the researchers were able to hone in on the gene P21. The protein produced by this gene previously had been demonstrated to protect cardiac cells from damage accrued by hypoxic conditions. The researchers used small molecules to decrease the signaling between Reverb alpha and this gene, finding that, after doing so, mice with heart cells damaged in the sleep-to-wake group fared as well as their counterparts that were injured later in their active period.
“If there is a specific role for Rev-erb alpha, it’s quite difficult to say,” said David Montaigne, the first author on the Lancet paper. “It’s one of the necessary parts of the circadian clock, so its absence would be quite problematic for the cell.” Montaigne and his colleagues hope to develop a drug based on the small molecule that could be used to minimize cell death and damage in human patients during strokes and heart attacks.
Clockwork’s edge
As a new generation of scientists continues to unravel the effects the circadian clocks have on human physiology, the Nobel laureates and other chronobiologists are investigating the near-invisible molecular interactions that cause circadian rhythm to operate in a 24-hour cycle.
 Michael Rosbash is the Peter Gruber endowed chair in neuroscience and a professor of biology at Brandeis University.courtesy of MIKE LOVETT
Michael Rosbash is the Peter Gruber endowed chair in neuroscience and a professor of biology at Brandeis University.courtesy of MIKE LOVETT
“We really, to this day, don’t understand how the clock runs at a 24-hour pace,” said Paul Hardin, a chronobiologist at Texas A&M University who helped demonstrate, while he was a postdoctoral researcher in Rosbash’s lab in the early ’90s, that mutant versions of the period gene affected PER mRNA transcription and the length of the circadian rhythm’s period. “What is important from here on out in model organisms is to understand how transcription is controlled, how those transcriptions control overt rhythms, and how that can be translated to bettering human health.”
Rosbash’s lab at Brandeis is investigating this molecular activity, as well as the clock’s ability to operate consistently in changing temperatures. Unlike many other enzymatic reactions, which tend to speed up when ambient temperature increases, he said, the mechanisms of the circadian rhythm in fruit flies haven’t been observed to accelerate within the ambient temperature range between 18 degrees and 29 degrees Celsius (64 degrees and 84.2 degrees Fahrenheit), an unexplained physiological anomaly.
“I think the two interesting frontiers, really, are the details of timing,” he said. “Why is a fruit fly’s period 23.8 hours and a human’s 24 hours and 15 minutes and not 22 or 2? And coupled to that is this terribly interesting problem of temperature compensation in circadian biology.”
The difference between the 24-hour solar cycle and the 24-hour–15-minute human circadian rhythm is subtle but crucial, Scheer said. “That may seem kind of trivial, the distinction, but of course, it has big influences on whether the circadian system can easily be entrained to the Earth day and also whether it could be entrained to other days, such as the cycle length on the planet Mars, which is 24.65 hours long.”
Young’s lab is exploring mutations in humans responsible for delayed sleep disorder, in addition to mutations in fruit flies that are responsible for the duration of sleep.
“Every time I hear someone say we’ve learned all we can from model organisms, I see something pop up that proves that we’ve just opened a new door,” Young said. “The work that was done in Drosophila so clearly set the path for the understanding of circadian biology in every system, and it’s impossible to imagine having started in a more complicated system and had the wherewithal to be where we are today.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

National Academies propose initiative to sequence all RNA molecules
Unlocking the epitranscriptome could transform health, medicine, agriculture, energy and national security.
From the journals: JLR
What can you do with artificial lipoproteins? A new key to angiogenesis. Flavonoids counteract oxidative stress. Read about recent papers on these topics.
Iron could be key to treating a global parasitic disease
A study has found that leishmaniasis causes body-wide changes in iron balance, leading to red blood cell damage.

Environmental DNA is everywhere
The ability to extract trace bits of DNA from soil, water, and even air is revolutionizing science. Are there pitfalls?
Early COVID-19 research is riddled with poor methods and low-quality results
The pandemic worsened, but didn’t create, this problem for science.

From the journals: MCP
Three views of mass spec: analyzing secreted protein spectra, imaging mass spectrometry for clinical use and spectral libraries for MS data analysis. Read about these recent papers.

