Nobel Prize in chemistry recognizes three DNA repair mechanisms
In the very act of being alive, we humans expose our DNA to a constant onslaught of damage. Every time it replicates, our genetic code amasses mutations. Ultraviolet rays from the sun, oxygen in our lungs, and exposure to natural and synthetic chemicals cause us to accumulate about 200 mutations per day. If not for certain repair mechanisms present in the human genome, our lives would be very short.
We now know that more than 100 genes are involved in the efficient correction of DNA damage. Problems with DNA repair mechanisms can have serious consequences, in some cases leading to cancer and other conditions, such as neurological damage and developmental defects. Changes to the infamous BRCA1 tumor suppressor gene, for example, can reduce the cell’s ability to repair DNA, leading to breast or ovarian cancer.
There was a time when scientists assumed DNA was immutable, but that time has passed. “Damage is all over the genome,” says F. Peter Guengerich of Vanderbilt University. “Even if you lead a relatively clean life, you’re still going to get certain amounts of damage.”
Guengerich is the interim editor-in-chief of the Journal of Biological Chemistry, which has published papers by the three biochemists who will be awarded Nobel Prizes this month for describing some of the chemistry of DNA repair. (The JBC is published by the American Society for Biochemistry and Molecular Biology.)
On Dec. 10, 2015, Tomas Lindahl, Paul Modrich and Aziz Sancar will receive the Nobel Prize in chemistry. All three are members of the ASBMB and contributors to a field of DNA repair that dates back to the late 1920s. (Scientists were studying the effects of radiation on the genome long before comprehending its structure.) And while none of this year’s laureates discovered DNA repair per se, each has made major contributions to the molecular understanding of how various repair pathways fix different types of DNA damage.
Therapeutic applications are beginning to emerge from our understanding of DNA repair mechanisms. This year’s Nobel Prize in chemistry highlights the value of fundamental research done at a time before translational applications were on scientists’ radar. Modrich appreciates the Nobel committee underlining the importance of basic research. “My personal view is that most major biomedical advances can be directly traced to advances in basic science,” he says.
Sancar will receive the Bert and Natalie Vallee Award in Biomedical Science, which recognizes established scientists for outstanding accomplishments in basic biomedical research, at the ASBMB 2016 Annual Meeting in San Diego next April.
Base excision
 LINDAHL
LINDAHL
Tomas Lindahl, now at the Clare Hall laboratory of the Francis Crick Institute in the U.K., carried out his prize-winning research at the Karolinska Institute in Stockholm. In the 1970s, he and his team demonstrated that DNA decays over time. This work eventually led Lindahl to identify the DNA repair mechanism known as base excision repair.
Base excision repair targets routine chemical damage that would otherwise cause breaks or mutations in the DNA – problems that could potentially lead to disease. This pathway seeks out and removes damaged bases in DNA strands and then patches the strands with new nucleotides.
Patrick Sung, a DNA repair expert at Yale University and a JBC associate editor, finds the Nobel committee’s acknowledgement “a major morale booster for people who study DNA repair,” and says, “Lindahl’s work has brought to the forefront of our awareness that DNA repair is critically important. He’s been a very powerful spokesperson for the field over several decades.”
Indeed, from 1986 to 2005, Lindahl served as the director for Clare Hall, the principal research laboratory of the Imperial Cancer Research Fund (now a part of Cancer Research UK). “My time at Clare Hall has been very stimulating,” he says. “We tried to get together a number of people who were top-class in the field of DNA repair, which wasn’t as fashionable at that time as it is now. In that way, the Clare Hall laboratory became a world leader in this area.”
Mismatch
 MODRICH
MODRICH
Paul Modrich, at the Howard Hughes Medical Institute and the Duke University School of Medicine, has worked extensively on DNA mismatch repair. The copying mechanism for DNA is imperfect: Each time a cell duplicates its DNA, incorrect bases sneak in, known as mismatches. Rather than allow these mistakes to perpetuate, the mismatch repair pathway acts as a copyeditor, seeking out the errors and fixing them. Errors are 1,000 times less likely with a functional mismatch repair system.
Modrich and colleagues determined the pathways for mismatch repair in both E. coli and human cells. They also demonstrated that tumor cells from patients with Lynch syndrome, one of the most common forms of hereditary cancer, are deficient in mismatch repair. They showed that such cancers have insufficient levels of certain proteins required for the initiation of mismatch repair.
In addition to his work on mismatch repair’s role in Lynch syndrome and related cancers, Modrich is interested in the expansion of triplet repeat sequences, which underlies a number of neurodegenerative diseases. In the case of Huntington’s disease, for example, the proliferation of a specific three-base DNA sequence leads to the production of toxic proteins in the brain. Work by others in mouse models has shown that triplet expansion depends upon a functional mismatch repair system.
Nucleotide excision
 SANCAR
SANCAR
Aziz Sancar, at the University of North Carolina, Chapel Hill, started studying how UV light can cause DNA damage in 1974. UV exposure causes lesions, such as the thymine dimer, which induce a kink in the DNA. During UV light exposure, a photon causes a covalent bond to form between two bases where none should exist.
Sancar and colleagues mapped out a pathway similar to Lindahl’s, known as nucleotide excision repair. Sancar’s research was based on the work he was doing with photolyase, an enzyme that repairs UV damage to DNA in certain organisms. He improved our understanding of the nucleotide excision repair pathway, which corrects lesions resulting from UV light exposure by detecting the damage, cutting it from the DNA, and rejoining the strands. This research has been instrumental to our understanding of skin cancer, which can be caused by UV light damage.
This year, Sancar and colleagues completed a map of the nucleotide excision repair pathway for the entire human genome. This map can be used to determine the ways in which each specific nucleotide in the genome is repaired. Sancar is gratified to have finished the project: “I went to Peru to give a couple lectures. I told my wife that if my plane hits the Andes and I die, I’ll die a happy man because I have the map.”
Cancer and clocks
As seen in the work of Modrich and others over the past 20 years, DNA repair is not always positive. Cancer cells with increased DNA repair activity can develop resistance to radiation or chemotherapy. For this reason, fundamental research into the various repair pathways is starting to make inroads toward clinical applications. Drugs that hinder DNA repair are currently undergoing clinical trials.
However, many aspects of DNA repair are highly complex and not yet fully understood. One example is the role of the circadian clock. In mammals, the circadian clock turns genes on and off depending on the time of day. Sancar and his colleagues found that DNA repair activity in mice is at its highest around 4 p.m. and at its lowest around 4 a.m. In cases where DNA repair can diminish the efficacy of chemotherapy, this fluctuation potentially could be exploited to time the delivery of anticancer drugs, optimizing their impact with minimal side effects.
Stimulating the field
There are many DNA repair mechanisms beyond the three acknowledged by the 2015 Nobel Prize in chemistry, such as chromosomal breakage repair. Many who made important contributions were not recognized due to the interpretation of specific stipulations in Alfred Nobel’s will by the prize committee. For example, Sancar’s mentor Claud Rupert discovered the enzyme photolyase in 1958. Sancar considers him to be the father of the field.
Nevertheless, experts in DNA repair are thrilled with the recognition bestowed by the prize. As Lindahl says, “I hope that it will stimulate the field a great deal. My colleagues are very positive and enthusiastic about drawing attention to the field.”
Royal Swedish Academy of Sciences’ explains Nobel Prize in chemistry 2015.
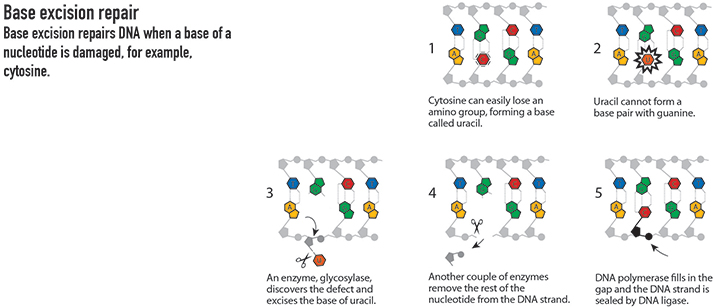

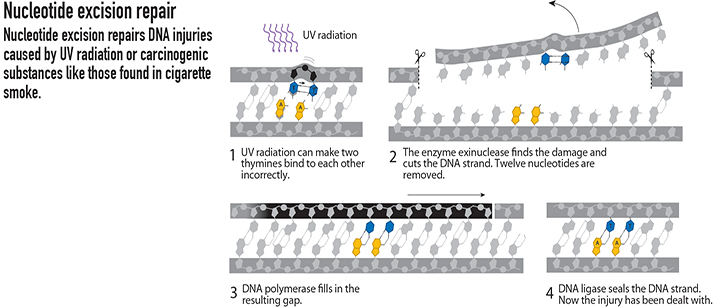 FROM THE ROYAL SWEDISH ACADEMY OF SCIENCES NOBEL PRIZE IN CHEMISTRY 2015 POPULAR SCIENCE BACKGROUND, ILLUSTRATIONS BY JOHAN JARNESTAD/THE ROYAL SWEDISH ACADEMY OF SCIENCES
FROM THE ROYAL SWEDISH ACADEMY OF SCIENCES NOBEL PRIZE IN CHEMISTRY 2015 POPULAR SCIENCE BACKGROUND, ILLUSTRATIONS BY JOHAN JARNESTAD/THE ROYAL SWEDISH ACADEMY OF SCIENCES
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Immune cells can adapt to invading pathogens
A team of bioengineers studies how T cells decide whether to fight now or prepare for the next battle.
Hinton lab maps structure of mitochondria at different life stages
An international team determines the differences in the 3D morphology of mitochondria and cristae, their inner membrane folds, in brown adipose tissue.

National Academies propose initiative to sequence all RNA molecules
Unlocking the epitranscriptome could transform health, medicine, agriculture, energy and national security.
From the journals: JLR
What can you do with artificial lipoproteins? A new key to angiogenesis. Flavonoids counteract oxidative stress. Read about recent papers on these topics.
Iron could be key to treating a global parasitic disease
A study has found that leishmaniasis causes body-wide changes in iron balance, leading to red blood cell damage.

Environmental DNA is everywhere
The ability to extract trace bits of DNA from soil, water, and even air is revolutionizing science. Are there pitfalls?


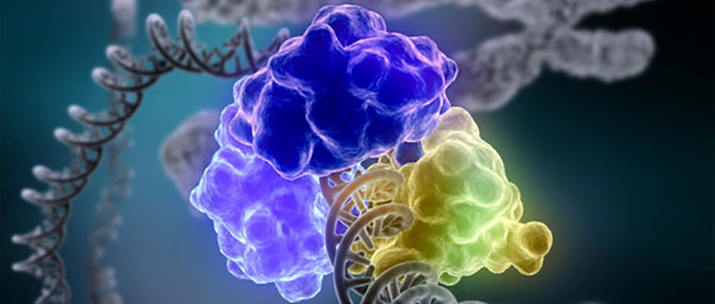 An enzyme encircles and repairs a broken DNA strand. IMAGE COURTESY OF TOM ELLENBERGER, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE IN ST. LOUIS, AND DAVE GOHARA, SAINT LOUIS UNIVERSITY SCHOOL OF MEDICINE (NATIONAL INSTITUTES OF HEALTH)
An enzyme encircles and repairs a broken DNA strand. IMAGE COURTESY OF TOM ELLENBERGER, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE IN ST. LOUIS, AND DAVE GOHARA, SAINT LOUIS UNIVERSITY SCHOOL OF MEDICINE (NATIONAL INSTITUTES OF HEALTH)