From the journals: January 2019
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
Fresh CRISPR-Cas delivery
The bacterial CRISPR-Cas9 system can be used to edit genes with single-nucleotide precision, but current methods of delivering the system remain inefficient in real-world therapeutic settings. Currently, most methods deliver nucleic acids encoding Cas9, which subsequently are translated in the cell. Delivering already synthesized Cas9 protein and guide RNA into cells would be a more direct approach. Yuefei Shen and colleagues at University of Massachusetts Medical School developed CRISPR delivery particles built on amphipathic peptides to do just that. As proof of concept in the Journal of Biological Chemistry, they used these particles to edit a gene in fat cells to convert white adipocytes into energy-burning brown adipocytes.
Polyunsaturated fatty acids in dividing T cells
As cells grow and prepare for cell division, they also synthesize the lipid components of the cell membrane, some of which do double duty as signaling molecules. However, mammals cannot synthesize from scratch all the fatty acids they need, such as most polyunsaturated fatty acids, or PUFAs. The initial building block for omega-3 and omega-6 PUFA is an 18-carbon fatty acid with two or three double bonds derived from the diet.
Once in cells, enzymes elongate and desaturate these 18-carbon PUFAs to generate even longer and more unsaturated PUFAs. While several enzymes have been shown to catalyze these reactions, which of them play a significant role in proliferating cells is unknown. In a paper published in the Journal of Lipid Research, Marc Surette and colleagues at the universities of Mancton and Quebec, Canada, characterized the PUFA profiles and the responsible enzymes in resting and proliferating primary human T cells and in the Jurkat cell line. The investigators found that both primary and cultured cells had a greater capacity to incorporate, elongate and desaturate exogenous PUFA when proliferating. Furthermore, they identified ELOVL5 as the key elongase in this process. Future studies will show whether changes in PUFA profiles are necessary for successful cell division and how PUFA misregulation contributes to diseases with proliferation defects, such as cancers.
The call is coming from outside the house
During nutrient shortages, cells’ nutrient sensors can trigger autophagy to recycle cellular components. This process is dysregulated in cancer and metabolic diseases. Maria Gubbiotti and colleagues at Thomas Jefferson University showed that an important nutrient sensor is located outside the cell, in the extracellular matrix. The extracellular proteoglycan decorin was required to induce autophagy in cardiomyocytes in fasting mice. This study, published in the Journal of Biological Chemistry, hints that the extracellular matrix doesn’t just hold cells in place but plays an active role in cellular metabolism.
Linking diabetes to cognitive decline
Chronic diabetes has a well-known yet little understood effect called cognitive decline. In fact, patients with diabetes are more likely to develop cognitive disorders such as dementia than those without. To determine the link between the two, Liangcai Zhao and colleagues at Wenzhou Medical University performed a metabolomics study on the brains of diabetic rats. This work was published in Molecular & Cellular Proteomics.
First, the researchers treated rats with the drug streptozotocin, which selectively attacks pancreatic beta cells, causing hyperglycemia and other symptoms associated with diabetes. After observing the cognitive decline in these rats using behavioral tests and brain scans, they looked at which metabolites changed with diabetes, specifically in the hippocampus. Lactate, a metabolite in the glycolysis pathway, was higher in the diabetic rats’ brains than control animals, especially when the disease became chronic.
While glycolysis generates some energy, cells usually move to the next step, the tricarboxylic acid cycle, to make even more energy. In chronic diabetic rats’ brains, however, cells use glycolysis for energy exclusively, and the enzyme used to make lactate had higher activity in these animals.
Not all of this lactate was utilized, however. Lactate transporter levels decreased during the chronic stage such that excess lactate accumulated between astrocytes, a type of brain cell with a range of important roles. The excess dysregulated a specific signaling pathway involved in the transcription of genes in learning and memory, thus providing a link between diabetes and cognitive decline. To alleviate lactate’s hold on cognitive decline, researchers blocked its production with an inhibitor. Diabetic rats showed improvement on behavioral tests, which indicated better memory, and brain scans showed less atrophy than previously observed.
The authors suggest that blocking the production of lactate in diabetic patients may be a way to limit cognitive decline.
— Dawn Hayward
 |
|
Wikimedia
|
|
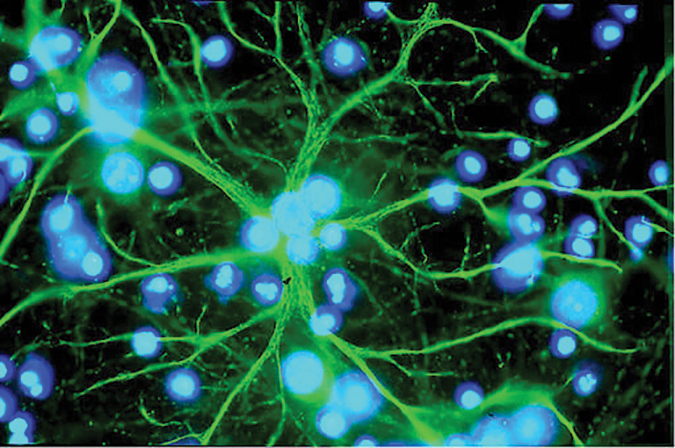
Astrocytes in the brain can accumulate the metabolite lactate between cells during chronic diabetes in rats, contributing to cognitive decline.
|
A new way to quantify your favorite protein
Tracking where a protein is inside a cell and exactly how much there is isn’t trivial. Scientists use mass spectrometry, antibodies, tags and other means to find and follow their favorite protein, but these methods cannot be used for real-time intracellular imaging. Chromobodies (nanobodies with a fluorescent tag) can be injected into cells and bind endogenous proteins. Bettina-Maria Keller and colleagues at the University of Tuebingen in Germany optimized a chromobody to improve its sensitivity in tracking endogenous proteins. The work, published in Molecular & Cellular Proteomics, introduces these destabilized chromobodies. To make the chromobody sensitive to time-dependent changes in concentration, researchers altered its N-terminal sequence so it was degraded immediately by the cell if it did not specifically bind its antigen. After pathway induction or drug treatment, the amount of chromobody, and therefore fluorescence, tracked the increases and decreases in protein concentration in real time. This new and improved chromobody now can be used instead of traditional methods to track a scientist’s favorite protein sensitively.
A new heme catabolite
Hydrogen sulfide is a gaseous signaling molecule important for many biological processes. Toshitaka Matsui and colleagues at Tohoku University report a new reaction pathway in which hydrogen sulfide induces heme oxygenase to produce new isomers of sulfur-containing biliverdin, or SBV, and describe the mechanism of the formation of these catabolites. Biliverdins, the bile pigments responsible for the greenish color of bruises, increasingly are recognized as antioxidants. The new SBV-producing pathway was less dependent on oxygen concentration than canonical heme oxygenase activation, leading the authors to speculate that it allows mammalian cells to degrade heme and produce antioxidants under hypoxic conditions. The study was published in the Journal of Biological Chemistry.
Cholesterol-lowering drug target discovered
About a quarter of deaths in the United States are caused by heart disease, according to the Centers for Disease Control and Prevention. Hypercholesterolemia, or high cholesterol, is a major risk factor for cardiovascular disease. However, cholesterol, a component of lipid bylayers, is essential for life. Mammals can either synthesize cholesterol or absorb it from food using the intestinal transmembrane protein Niemann–Pick C1-like 1, or NPC1L1. This transporter resides in lipid rafts, which are membrane microdomains used for cell–cell interaction and cell signaling that are enriched in cholesterol as well as gangliosides — a group of galactose-containing glycolipids. In a paper in the Journal of Lipid Research, Jin-ichi Inokuchi from Tohoku University in Japan and colleagues show that NPC1L1-dependent intestinal cholesterol uptake requires the ganglioside GM3 and the enzyme that synthesizes it, GM3S. Cholesterol uptake is decreased in GM3S-deficient cells, and GM3S-deficient mice fed a high-cholesterol diet show a lower susceptibility to hypercholesterolemia. This research proposes a new viable target for cholesterol reducing therapies.
 |
|
Brocker et al./JLR
|
|
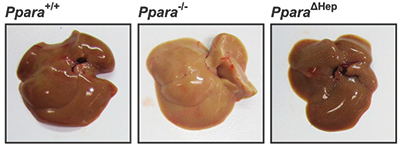
Mice lacking PPARA in the entire body develop abnormal lipid accumulation in the liver during fasting, pictured in the center, which makes the liver appear paler than a normal liver, at left. When PPAR is missing only in the liver, at right, this phenotype is ameliorated. To survive fasting, humans and other mammals can shift their metabolism from reliance on glucose and fat derived from food to reliance on fat stores. Peroxisome proliferator-activated receptor alpha, or PPARA, is a major regulator of lipid homeostasis and is critical for surviving fasting and starvation. PPARA, a transcription factor found in the liver and some other tissues, upregulates genes that contribute to the catabolism of free fatty acids, or FFAs.
|
Protecting the liver — a full-body job
In a paper published in the Journal of Lipid Research, Frank Gonzalez and his team at the National Cancer Institute together with collaborators in China demonstrated that PPARA in tissues outside the liver can protect a liver that lacks PPARA during fasting. The investigators compared the liver phenotype of normal mice and mice lacking PPARA either in the entire body or only in the liver after one day of fasting.
Food deprivation is associated with elevated fat-derived FFAs circulating in the blood, which are taken up by the liver. In normal mice, these FFAs are broken down through the tricarboxylic acid cycle to generate energy. Mice completely lacking PPARA still can uptake FFAs into the liver but cannot catabolize them, leading to an abnormal lipid accumulation in the liver called hepatosteatosis. In mice lacking PPARA in only the liver, the researchers found that this phenotype was dramatically redced. PPARA activity from outside the liver boosted fatty acid oxidation and lipase activity, reduced the systemic lipid load and reduced lipid accumulation in the liver.
PPARA function is decreased in several diseases affecting the liver such as nonalcoholic fatty liver disease and hepatitis C. This research suggests that PPARA from outside the liver could compensate for low hepatic PPARA, which may help develop novel approaches to treat these diseases.
— Nathalie Gerassimov
Crystals, cataracts and hot potatoes
Highly stable crystallin proteins in the lens of the human eye are never replaced over a lifetime. With age, though, they accumulate oxidation-induced disulfide bonds, which can lead to formation of protein aggregates and cataract disease. Eugene Serebryany and colleagues at Harvard University examined disulfide bond formation in mixtures of wild-type and cataract-associated human γD-crystallin. They identify a “redox hot potato competition” wherein the disulfide bonds are exchanged dynamically between molecules and can be trapped in aggregation-prone intermediates. This study, published in the Journal of Biological Chemistry, thus reveals a new enzyme function for crystallins and provides potential insights into the evolution of exceptionally long-lived proteins.
Decorating bacterial walls
The teichoic acids in the cell walls of Gram-positive bacteria are modified frequently, altering bacterial growth, aggregation and resistance to antibiotics. Understanding how these modifications are introduced is therefore important for combating these bacteria, but the pathways are difficult to characterize. In the Journal of Biological Chemistry, B. McKay Wood and colleagues at Harvard Medical School write that they developed a suite of assays, including a partially reconstituted model system, to examine steps in the teichoic acid D-alanylation pathway. They found that a previously uncharacterized membrane protein in the pathway attaches alanine onto lipotechoic acid, defining one new step and setting the stage for further investigations.
Why a pain drug failed in humans
Everyone knows that humans aren’t rats, but in the context of preclinical laboratory studies, we’ve got to do our best with what we’ve got. Many drugs show promise during rodent trials and subsequently fail in humans. To make such studies more efficient, it’s important to understand how humans differ from model species at the molecular sites that potential drugs target.
Neuropathic pain is caused by the nervous system misfiring rather than by stimulation of typical pain receptors. Potential drug targets for neuropathic pain are the nicotinic acetylcholine receptors, or nAChRs, in the dorsal root ganglia. However, the sensitivity of receptors such as nAChRs differs in rodents and humans. In a recent example, a conotoxin peptide — a venom produced by carnivorous marine snails — alleviated neuropathic pain in mice but not in people.
In a study published in the Journal of Biological Chemistry, Arik J. Hone and colleagues at the University of Utah investigated the molecular basis of this difference in effectiveness between conotoxin inhibition of human and rat nAChRs. They found that, on the whole, the pocket on the receptor that binds ligands like conotoxins looked very similar in the two species.
But three amino acids were different, and one of these differences was critical: The substituted amino acid slightly changed the orientation of the conotoxin as it attempted to bind the human receptor, resulting in reduced potency. Changing the same amino acid in the rat receptor did not affect its potency, suggesting that differences elsewhere in the receptor also affected how the receptor and ligand interacted.
Understanding the molecular details of species-specific drug targets may help researchers to better predict which pharmacological findings will be translatable from animal models to humans.
— Sasha Mushegian
 |
|
Wikipedia
|
|
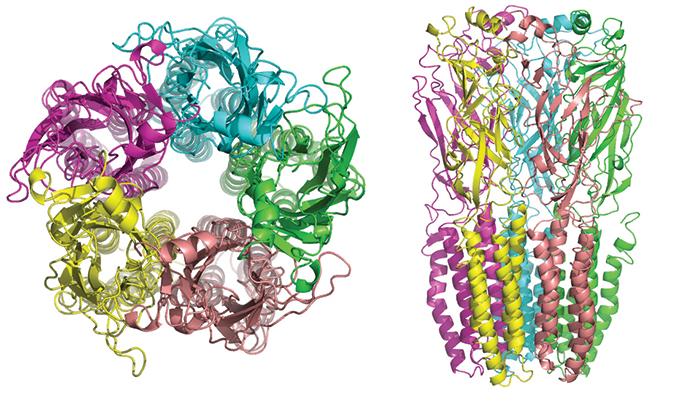
The five subunits of nicotinic receptors are arranged symmetrically around a central pore, and each subunit comprises four transmembrane domains with both the N- and C-terminus located extracellularly.
|
Negative effects of controlled malaria in pregnancy
Malaria during pregnancy can result in detrimental fetal effects, such as low birth weight. After parasite infection, the mother’s immune response restricts parasitic growth to the placenta, but chemokines and other immune cells can infiltrate and prevent nutrient flow to the fetus. Clearance by drug treatment, while helpful, does not reverse all fetal effects. Rebeca Kawahara and colleagues at the University of Sao Paulo in Brazil did a proteomics study on placental tissues from uninfected and previously infected mothers and used a mouse model to look at cellular changes in controlled placental malaria. This work was published in Molecular & Cellular Proteomics. Using mass spectrometry and statistical analysis, they identified proteins with altered expression levels, their associated cellular processes and post-translational modifications indicative of changes in regulation. Mapping the resultant networks revealed that in the placenta of the previously infected mothers, processes such as apoptosis, cell signaling and oxidative stress were dysregulated, an observation also seen in the mouse model. This means that, although the infection was cleared, placental cells could face cell death, alteration of intracellular pathways and increasing cellular stress. The authors say that these pathways, though harmful to the placenta and fetus, could be used as markers in the clinic, making it easier to detect the effects of malaria in pregnancy.
Regulation by membrane curvature
Some proteins can be found in both the plasma membrane and the endoplasmic reticulum membrane. One example is diacylglycerol kinase epsilon, or DGK-epsilon, which carries out important functions in the phosphatidylinositol cycle. José Carlos Bozelli and colleagues at McMaster University investigated whether DGK-epsilon activity is affected by the properties of the membranes in which they can be embedded. They found that, when embedded in a locally flat membrane, the kinase had low activity and broad acyl chain specificity; curved membranes, in contrast, improved activity and specificity by allosterically regulating DGK-epsilon activity. Thus, membrane shape, in addition to lipid composition, may be an important regulator of lipid signaling. The study was published in the Journal of Biological Chemistry.
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Iron could be key to treating a global parasitic disease
A study has found that leishmaniasis causes body-wide changes in iron balance, leading to red blood cell damage.

Environmental DNA is everywhere
The ability to extract trace bits of DNA from soil, water, and even air is revolutionizing science. Are there pitfalls?
Early COVID-19 research is riddled with poor methods and low-quality results
The pandemic worsened, but didn’t create, this problem for science.

From the journals: MCP
Three views of mass spec: analyzing secreted protein spectra, imaging mass spectrometry for clinical use and spectral libraries for MS data analysis. Read about these recent papers.

Understanding the fat science
Researchers at UCLA investigate lipid remodeling in the liver for energy generation.

No oxygen? No problem
By studying how electric fish survive in hypoxic streams for months at time, researchers may find new ways to target tumors.



.jpg?lang=en-US&width=300&height=300&ext=.jpg)